当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Intermolecular Electrophilic Bromoesterification and Bromoetherification of Unactivated Cyclopropanes
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2020-03-23 , DOI: 10.1002/adsc.201901665 Vincent Ming‐Yau Leung 1 , Matthew H. Gieuw 1 , Zhihai Ke 1 , Ying‐Yeung Yeung 1
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2020-03-23 , DOI: 10.1002/adsc.201901665 Vincent Ming‐Yau Leung 1 , Matthew H. Gieuw 1 , Zhihai Ke 1 , Ying‐Yeung Yeung 1
Affiliation
|
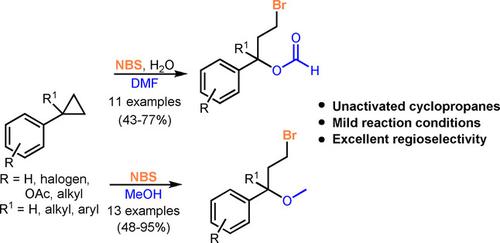
1,3‐difunctionalization of cyclopropane is an useful organic transformation. The corresponding 1,3‐difunctionalized products are synthetic synthons and building blocks in many organic syntheses. Many existing ring‐opening difunctionalization methodologies rely primarily on the use of donor−acceptor cyclopropanes, while the difunctionalization of unactivated cyclopropanes is less exploited. In this research, 1,3‐bromoesterification and 1,3‐bromoetherification of unactivated cyclopropanes were successfully achieved using N‐bromosuccinimide as the brominating agent with high yields and regioselectivity.
中文翻译:

分子间亲电溴化和未活化环丙烷的溴醚化
环丙烷的1,3-二官能化是一种有用的有机转化方法。相应的1,3-双功能化产品是许多有机合成中的合成合成子和构件。许多现有的开环双官能化方法主要依靠供体-受体环丙烷的使用,而未活化的环丙烷的双官能化的利用较少。在这项研究中,使用N-溴代琥珀酰亚胺作为溴化剂,以高收率和区域选择性成功地实现了未活化的环丙烷的1,3-溴代酯化和1,3-溴代醚化。
更新日期:2020-03-23
中文翻译:

分子间亲电溴化和未活化环丙烷的溴醚化
环丙烷的1,3-二官能化是一种有用的有机转化方法。相应的1,3-双功能化产品是许多有机合成中的合成合成子和构件。许多现有的开环双官能化方法主要依靠供体-受体环丙烷的使用,而未活化的环丙烷的双官能化的利用较少。在这项研究中,使用N-溴代琥珀酰亚胺作为溴化剂,以高收率和区域选择性成功地实现了未活化的环丙烷的1,3-溴代酯化和1,3-溴代醚化。


























 京公网安备 11010802027423号
京公网安备 11010802027423号