当前位置:
X-MOL 学术
›
Eur. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A novel approach to highly substituted β-carbolines via reductive ring transformation of 2-acyl-3-isoxazolylindoles
European Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2020-05-05 , DOI: 10.1002/ejoc.202000230 Alexandra Kamlah 1 , Franz Bracher 1
European Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2020-05-05 , DOI: 10.1002/ejoc.202000230 Alexandra Kamlah 1 , Franz Bracher 1
Affiliation
|
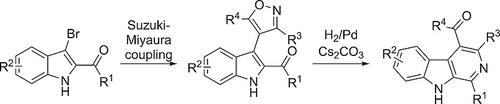
We have worked out a new approach to 1,3,4‐trisubstituted β‐carbolines of pharmaceutical interest. As central building blocks we used 2‐acylindoles, which are readily available from indole‐2‐Weinreb amides. Bromination at C‐3, followed by Suzuki–Miyaura cross‐coupling with isoxazole‐4‐boronates gives 2‐acyl‐3‐isoxazolylindoles. Ring closure to the β‐carbolines was accomplished by reductive ring transformation upon catalytic hydrogenation in the presence of Cs2CO3.
中文翻译:

通过 2-酰基-3-异恶唑基吲哚的还原环转化制备高度取代的 β-咔啉的新方法
我们已经制定出一种新方法来处理具有药用价值的 1,3,4-三取代 β-咔啉。我们使用 2-酰基吲哚作为中心构建块,它很容易从吲哚-2-Weinreb 酰胺中获得。在 C-3 处溴化,然后 Suzuki-Miyaura 与异恶唑-4-硼酸酯交叉偶联得到 2-酰基-3-异恶唑基吲哚。β-咔啉的闭环是通过在 Cs2CO3 存在下催化氢化时的还原环转化来实现的。
更新日期:2020-05-05
中文翻译:

通过 2-酰基-3-异恶唑基吲哚的还原环转化制备高度取代的 β-咔啉的新方法
我们已经制定出一种新方法来处理具有药用价值的 1,3,4-三取代 β-咔啉。我们使用 2-酰基吲哚作为中心构建块,它很容易从吲哚-2-Weinreb 酰胺中获得。在 C-3 处溴化,然后 Suzuki-Miyaura 与异恶唑-4-硼酸酯交叉偶联得到 2-酰基-3-异恶唑基吲哚。β-咔啉的闭环是通过在 Cs2CO3 存在下催化氢化时的还原环转化来实现的。


























 京公网安备 11010802027423号
京公网安备 11010802027423号