当前位置:
X-MOL 学术
›
Inorg. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Electrochemical behavior of NiCl2/Ni in acidic AlCl3-based ionic liquid electrolyte
Inorganic Chemistry Frontiers ( IF 7 ) Pub Date : 2020-03-23 , DOI: 10.1039/d0qi00166j Jiguo Tu 1, 2, 3, 4 , Mingyin Kou 2, 3, 4, 5 , Mingyong Wang 1, 2, 3, 4 , Shuqiang Jiao 1, 2, 3, 4
Inorganic Chemistry Frontiers ( IF 7 ) Pub Date : 2020-03-23 , DOI: 10.1039/d0qi00166j Jiguo Tu 1, 2, 3, 4 , Mingyin Kou 2, 3, 4, 5 , Mingyong Wang 1, 2, 3, 4 , Shuqiang Jiao 1, 2, 3, 4
Affiliation
|
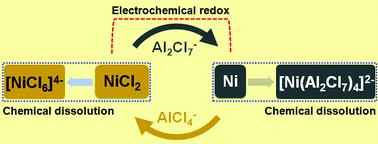
The electrochemical properties of nickel chloride as a cathode in basic electrolyte have been much examined, but little study has focused on those properties in acidic electrolyte. In this study, the electrochemical behavior of Al-NiCl2 in acidic AlCl3-EMIC ionic liquid electrolyte is investigated, confirming the reversible reaction between Ni(2) and Ni. The assembled cell delivers an initial discharge capacity of 115.0 mA h g−1 at 100 mA g−1, and it remains at 93.7 mA h g−1 over 100 cycles, with a Coulombic efficiency of 97.9%, displaying a high specific capacity with stable cycling performance. Additionally, the degradation mechanism of this cell is verified as the dissolution of partial Ni metal generated from the reduction of NiCl2 during discharging in the acidic electrolyte.
中文翻译:

NiCl2 / Ni在酸性AlCl3基离子液体电解质中的电化学行为
已经对作为碱性电解质中阴极的氯化镍的电化学性质进行了广泛的研究,但很少有研究集中在酸性电解质中。在这项研究中,研究了Al-NiCl 2在酸性AlCl 3 -EMIC离子液体电解质中的电化学行为,证实了Ni(2)和Ni之间可逆的反应。组装好的电池在100 mA g -1时提供115.0 mA hg -1的初始放电容量,并保持在93.7 mA hg -1处超过100个循环,库仑效率为97.9%,显示出高比容量和稳定的循环性能。另外,该电池的降解机理被证实为在酸性电解质中放电期间由NiCl 2的还原产生的部分Ni金属的溶解。
更新日期:2020-03-23
中文翻译:

NiCl2 / Ni在酸性AlCl3基离子液体电解质中的电化学行为
已经对作为碱性电解质中阴极的氯化镍的电化学性质进行了广泛的研究,但很少有研究集中在酸性电解质中。在这项研究中,研究了Al-NiCl 2在酸性AlCl 3 -EMIC离子液体电解质中的电化学行为,证实了Ni(2)和Ni之间可逆的反应。组装好的电池在100 mA g -1时提供115.0 mA hg -1的初始放电容量,并保持在93.7 mA hg -1处超过100个循环,库仑效率为97.9%,显示出高比容量和稳定的循环性能。另外,该电池的降解机理被证实为在酸性电解质中放电期间由NiCl 2的还原产生的部分Ni金属的溶解。


























 京公网安备 11010802027423号
京公网安备 11010802027423号