当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Efficient diversification of GM3 gangliosides via late-stage sialylation and dynamic glycan structural studies with 19F solid-state NMR.
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2020-04-15 , DOI: 10.1039/d0ob00437e Maina Takahashi 1 , Junya Shirasaki 2 , Naoko Komura 2 , Katsuaki Sasaki 3 , Hide-Nori Tanaka 2 , Akihiro Imamura 1 , Hideharu Ishida 4 , Shinya Hanashima 3 , Michio Murata 3 , Hiromune Ando 2
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2020-04-15 , DOI: 10.1039/d0ob00437e Maina Takahashi 1 , Junya Shirasaki 2 , Naoko Komura 2 , Katsuaki Sasaki 3 , Hide-Nori Tanaka 2 , Akihiro Imamura 1 , Hideharu Ishida 4 , Shinya Hanashima 3 , Michio Murata 3 , Hiromune Ando 2
Affiliation
|
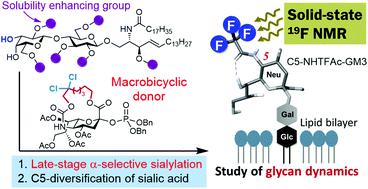
Sialic acid-containing glycoconjugates are involved in important biological processes such as immune response, cancer metastasis, and viral infection. However, their chemical syntheses have been challenging, mainly due to the difficulties in the α-sialylation of oligosaccharides. Very recently, we established a completely stereoselective sialidation method using a macrobicyclic sialyl donor. Herein, we describe a rational and efficient synthesis of sialoglycolipids via direct sialylation of a glycolipid at a late-stage, based on our novel sialidation method. The synthetic method enabled the development of GM3 ganglioside analogs with various C5-modifications of the sialosyl moiety. Furthermore, the synthesized analog was subjected to solid-state 19F NMR analysis on the model membranes and it revealed the influence of cholesterol on glycan dynamics.
中文翻译:

GM3神经节苷脂通过后期唾液酸化和19F固态NMR进行的动态聚糖结构研究可实现高效多样化。
含唾液酸的糖缀合物参与重要的生物学过程,例如免疫应答,癌症转移和病毒感染。然而,它们的化学合成一直具有挑战性,这主要是由于寡糖的α-唾液酸化困难。最近,我们建立了使用大双环唾液酸供体的完全立体选择性唾液酸化方法。在本文中,我们基于新的唾液酸化方法,描述了在后期通过糖脂的直接唾液酸化来合理有效地合成唾液糖脂的方法。合成方法能够开发具有唾液酸基部分的各种C5修饰的GM3神经节苷脂类似物。此外,合成的类似物在模型膜上进行了固态19F NMR分析,揭示了胆固醇对聚糖动力学的影响。
更新日期:2020-04-24
中文翻译:

GM3神经节苷脂通过后期唾液酸化和19F固态NMR进行的动态聚糖结构研究可实现高效多样化。
含唾液酸的糖缀合物参与重要的生物学过程,例如免疫应答,癌症转移和病毒感染。然而,它们的化学合成一直具有挑战性,这主要是由于寡糖的α-唾液酸化困难。最近,我们建立了使用大双环唾液酸供体的完全立体选择性唾液酸化方法。在本文中,我们基于新的唾液酸化方法,描述了在后期通过糖脂的直接唾液酸化来合理有效地合成唾液糖脂的方法。合成方法能够开发具有唾液酸基部分的各种C5修饰的GM3神经节苷脂类似物。此外,合成的类似物在模型膜上进行了固态19F NMR分析,揭示了胆固醇对聚糖动力学的影响。


























 京公网安备 11010802027423号
京公网安备 11010802027423号