Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Ca2+ participating self-assembly of an apoferritin nanostructure for nucleic acid drug delivery
Nanoscale ( IF 6.7 ) Pub Date : 2020/03/24 , DOI: 10.1039/d0nr00547a Haiqin Huang 1, 2, 3, 4 , Kang Sha 1, 2, 3, 4 , Hanitrarimalala Veroniaina 1, 2, 3, 4 , Ziheng Wu 5, 6, 7, 8, 9 , Zhenghong Wu 1, 2, 3, 4 , Xiaole Qi 1, 2, 3, 4, 5
Nanoscale ( IF 6.7 ) Pub Date : 2020/03/24 , DOI: 10.1039/d0nr00547a Haiqin Huang 1, 2, 3, 4 , Kang Sha 1, 2, 3, 4 , Hanitrarimalala Veroniaina 1, 2, 3, 4 , Ziheng Wu 5, 6, 7, 8, 9 , Zhenghong Wu 1, 2, 3, 4 , Xiaole Qi 1, 2, 3, 4, 5
Affiliation
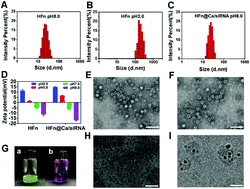
|
One of the most encountered obstacles for utilizing nano-sized vehicles to implement the in vivo delivery of nucleic acid drugs (NADs) is the possible steric hindrance caused by their intrinsic size and charge. In this work, we added Ca2+ for the pH triggered self-assembly process of H-apoferritin (HFn), to neutralize negative charges and help siRNA condense during complexation and particle formation. As expected, the internalization efficiency of siRNA in HFn particle formation could be enhanced 1.65-fold, compared with that without incorporated Ca2+. Furthermore, the calcification that occurred within the cavity of HFn particles endows them with endosomal escape capability, which could explain their contribution to the demonstrated in vitro and in vivo gene silencing effect achieved by the internalized siRNA. Thus, this Ca2+ participating self-assembly process of a protein nanostructure would lead to advanced internalization efficiency for NAD therapy.
中文翻译:

钙铁蛋白纳米结构的Ca2 +参与自组装,用于核酸药物递送
利用纳米媒介物实现核酸药物(NADs)体内输送的最常见障碍之一是其固有尺寸和电荷可能引起的空间位阻。在这项工作中,我们为H-铁蛋白(HFn)的pH触发自组装过程添加了Ca 2+,以中和负电荷并帮助siRNA在络合和颗粒形成过程中凝聚。不出所料,与未掺入Ca 2+相比,siRNA在HFn颗粒形成中的内在化效率可提高1.65倍。此外,HFn颗粒腔内发生的钙化使它们具有内体逃逸能力,这可以解释其对体外证明的作用和体内基因沉默效应被内在化的siRNA来实现。因此,这种Ca 2+参与的蛋白质纳米结构自组装过程将导致NAD治疗的更高的内在化效率。
更新日期:2020-04-03
中文翻译:

钙铁蛋白纳米结构的Ca2 +参与自组装,用于核酸药物递送
利用纳米媒介物实现核酸药物(NADs)体内输送的最常见障碍之一是其固有尺寸和电荷可能引起的空间位阻。在这项工作中,我们为H-铁蛋白(HFn)的pH触发自组装过程添加了Ca 2+,以中和负电荷并帮助siRNA在络合和颗粒形成过程中凝聚。不出所料,与未掺入Ca 2+相比,siRNA在HFn颗粒形成中的内在化效率可提高1.65倍。此外,HFn颗粒腔内发生的钙化使它们具有内体逃逸能力,这可以解释其对体外证明的作用和体内基因沉默效应被内在化的siRNA来实现。因此,这种Ca 2+参与的蛋白质纳米结构自组装过程将导致NAD治疗的更高的内在化效率。


























 京公网安备 11010802027423号
京公网安备 11010802027423号