当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The mechanism of Mg2+ conduction in ammine magnesium borohydride promoted by a neutral molecule.
Physical Chemistry Chemical Physics ( IF 3.3 ) Pub Date : 2020-03-31 , DOI: 10.1039/d0cp00158a Yigang Yan 1 , Wilke Dononelli , Mathias Jørgensen , Jakob B Grinderslev , Young-Su Lee , Young Whan Cho , Radovan Černý , Bjørk Hammer , Torben R Jensen
Physical Chemistry Chemical Physics ( IF 3.3 ) Pub Date : 2020-03-31 , DOI: 10.1039/d0cp00158a Yigang Yan 1 , Wilke Dononelli , Mathias Jørgensen , Jakob B Grinderslev , Young-Su Lee , Young Whan Cho , Radovan Černý , Bjørk Hammer , Torben R Jensen
Affiliation
|
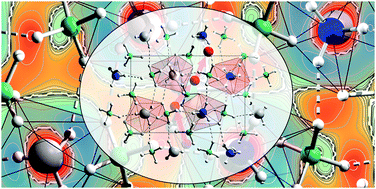
Light weight and cheap electrolytes with fast multi-valent ion conductivity can pave the way for future high-energy density solid-state batteries, beyond the lithium-ion battery. Here we present the mechanism of Mg-ion conductivity of monoammine magnesium borohydride, Mg(BH4)2·NH3. Density functional theory calculations (DFT) reveal that the neutral molecule (NH3) in Mg(BH4)2·NH3 is exchanged between the lattice and interstitial Mg2+ facilitated by a highly flexible structure, mainly owing to a network of di-hydrogen bonds, N-Hδ+-δH-B and the versatile coordination of the BH4- ligand. DFT shows that di-hydrogen bonds in inorganic matter and hydrogen bonds in bio-materials have similar bond strengths and bond lengths. As a result of the high structural flexibiliy, the Mg-ion conductivity is dramatically improved at moderate temperature, e.g. σ(Mg2+) = 3.3 × 10-4 S cm-1 at T = 80 °C for Mg(BH4)2·NH3, which is approximately 8 orders of magnitude higher than that of Mg(BH4)2. Our results may inspire a new approach for the design and discovery of unprecedented multivalent ion conductors.
中文翻译:

中性分子促进氨化硼氢化镁中Mg2 +的传导机制。
具有快速多价离子电导率的轻质,廉价电解质可以为锂离子电池以外的未来高能量密度固态电池铺平道路。在这里,我们介绍了单胺硼氢化镁Mg(BH4)2·NH3的Mg离子电导率的机理。密度泛函理论计算(DFT)显示,Mg(BH4)2·NH3中的中性分子(NH3)在晶格和间隙Mg2 +之间交换,这是由于其高度柔性的结构所致,这主要归因于二氢键N -Hδ+-δH-B和BH4-配体的多功能配位。DFT显示,无机物中的二氢键和生物材料中的氢键具有相似的键强度和键长。由于具有较高的结构柔韧性,因此在中等温度下(例如,对于Mg(BH4)2·NH3,在T = 80°C时,σ(Mg2 +)= 3.3×10-4 S cm-1,比Mg(BH4)2高出约8个数量级。我们的结果可能会激发出一种设计和发现空前的多价离子导体的新方法。
更新日期:2020-03-31
中文翻译:

中性分子促进氨化硼氢化镁中Mg2 +的传导机制。
具有快速多价离子电导率的轻质,廉价电解质可以为锂离子电池以外的未来高能量密度固态电池铺平道路。在这里,我们介绍了单胺硼氢化镁Mg(BH4)2·NH3的Mg离子电导率的机理。密度泛函理论计算(DFT)显示,Mg(BH4)2·NH3中的中性分子(NH3)在晶格和间隙Mg2 +之间交换,这是由于其高度柔性的结构所致,这主要归因于二氢键N -Hδ+-δH-B和BH4-配体的多功能配位。DFT显示,无机物中的二氢键和生物材料中的氢键具有相似的键强度和键长。由于具有较高的结构柔韧性,因此在中等温度下(例如,对于Mg(BH4)2·NH3,在T = 80°C时,σ(Mg2 +)= 3.3×10-4 S cm-1,比Mg(BH4)2高出约8个数量级。我们的结果可能会激发出一种设计和发现空前的多价离子导体的新方法。



























 京公网安备 11010802027423号
京公网安备 11010802027423号