JAMA Neurology ( IF 29.0 ) Pub Date : 2020-06-01 , DOI: 10.1001/jamaneurol.2020.0367 Merit Cudkowicz 1 , Marianne K Chase 1 , Christopher S Coffey 2 , Dixie J Ecklund 2 , Brenda J Thornell 1 , Codrin Lungu 3 , Katy Mahoney 1 , Laurie Gutmann 2 , Jeremy M Shefner 4 , Kevin J Staley 1 , Michael Bosch 2 , Eric Foster 2 , Jeffrey D Long 2 , Emine O Bayman 2 , James Torner 2 , Jon Yankey 2 , Richard Peters 2 , Trevis Huff 2 , Robin A Conwit 3 , , Shlomo Shinnar 5 , Donna Patch 5 , Basil T Darras 6 , Audrey Ellis 6 , Roger J Packer 7 , Karen S Marder 8, 9 , Claudia A Chiriboga 8, 9 , Claire Henchcliffe 8, 9 , Joyce Ann Moran 8, 9 , Blagovest Nikolov 8, 9 , Stewart A Factor 10 , Carole Seeley 10 , Steven M Greenberg 1, 11 , Anthony A Amato 1, 11 , Sara DeGregorio 1, 11 , Tanya Simuni 12 , Tina Ward 12 , John T Kissel 13 , Stephen J Kolb 13 , Amy Bartlett 13 , Joseph F Quinn 14 , Kellie Keith 14 , Steven R Levine 15 , Nadege Gilles 15 , Patricia K Coyle 16 , Jessica Lamb 16 , Gil I Wolfe 17 , Annemarie Crumlish 17 , Luis Mejico 18 , Muhammad Maaz Iqbal 18 , James D Bowen 19 , Caryl Tongco 19 , Louis B Nabors 20 , Khurram Bashir 20 , Melanie Benge 20 , Craig M McDonald 21 , Erik K Henricson 21 , Björn Oskarsson 21 , Bruce H Dobkin 22 , Catherine Canamar 22 , Tracy A Glauser 23 , Daniel Woo 23 , Angela Molloy 23 , Peggy Clark 23 , Timothy L Vollmer 24 , Alexander J Stein 24 , Richard J Barohn 25 , Mazen M Dimachkie 25 , Jean-Baptiste Le Pichon 25 , Michael G Benatar 26 , Julie Steele 26 , Lawrence Wechsler 27 , Paula R Clemens 27 , Christine Amity 27 , Robert G Holloway 28 , Christine Annis 28 , Mark P Goldberg 29 , Mariam Andersen 29 , Susan T Iannaccone 29 , A Gordon Smith 30 , J Robinson Singleton 30 , Mariana Doudova 30 , E Clarke Haley 31 , Mark S Quigg 31 , Stephanie Lowenhaupt 31 , Beth A Malow 32 , Karen Adkins 32 , David B Clifford 33 , Mengesha A Teshome 33 , Noreen Connolly 28
|
Importance One major advantage of developing large, federally funded networks for clinical research in neurology is the ability to have a trial-ready network that can efficiently conduct scientifically rigorous projects to improve the health of people with neurologic disorders.
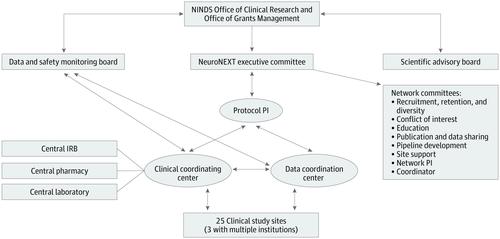
Observations National Institute of Neurological Disorders and Stroke Network for Excellence in Neuroscience Clinical Trials (NeuroNEXT) was established in 2011 and renewed in 2018 with the goal of being an efficient network to test between 5 and 7 promising new agents in phase II clinical trials. A clinical coordinating center, data coordinating center, and 25 sites were competitively chosen. Common infrastructure was developed to accelerate timelines for clinical trials, including central institutional review board (a first for the National Institute of Neurological Disorders and Stroke), master clinical trial agreements, the use of common data elements, and experienced research sites and coordination centers. During the first 7 years, the network exceeded the goal of conducting 5 to 7 studies, with 9 funded. High interest was evident by receipt of 148 initial applications for potential studies in various neurologic disorders. Across the first 8 studies (the ninth study was funded at end of initial funding period), the central institutional review board approved the initial protocol in a mean (SD) of 59 (21) days, and additional sites were added a mean (SD) of 22 (18) days after submission. The median time from central institutional review board approval to first site activation was 47.5 days (mean, 102.1; range, 1-282) and from first site activation to first participant consent was 27 days (mean, 37.5; range, 0-96). The median time for database readiness was 3.5 months (mean, 4.0; range, 0-8) from funding receipt. In the 4 completed studies, enrollment met or exceeded expectations with 96% overall data accuracy across all sites. Nine peer-reviewed manuscripts were published, and 22 oral presentations or posters and 9 invited presentations were given at regional, national, and international meetings.
Conclusions and Relevance NeuroNEXT initiated 8 studies, successfully enrolled participants at or ahead of schedule, collected high-quality data, published primary results in high-impact journals, and provided mentorship, expert statistical, and trial management support to several new investigators. Partnerships were successfully created between government, academia, industry, foundations, and patient advocacy groups. Clinical trial consortia can efficiently and successfully address a range of important neurologic research and therapeutic questions.
中文翻译:

美国国家神经病学研究所和中风支持的神经科学临床试验网络的七年经验。
重要性 开发大型的,由联邦政府资助的神经病学临床研究网络的一个主要优势是能够拥有一个可以随时进行试验的网络,该网络可以有效地进行严格的科学计划,以改善神经系统疾病患者的健康。
观察结果 美国国家神经疾病研究所和卒中神经科学临床试验卓越网络(NeuroNEXT)成立于2011年,并于2018年进行了更新,其目标是成为有效的网络,以在II期临床试验中测试5至7种有希望的新药。竞争性地选择了临床协调中心,数据协调中心和25个站点。通用基础设施的开发是为了加快临床试验的时间表,包括中央机构评审委员会(美国国家神经疾病和中风研究所的首创),主要的临床试验协议,通用数据元素的使用以及经验丰富的研究中心和协调中心。在最初的7年中,该网络超出了进行5到7项研究的目标,其中9项获得了资助。收到148份针对各种神经系统疾病的潜在研究的初始申请,表明了人们的高度兴趣。在前8项研究中(第9项研究在初始资助期结束时获得资助),中央机构审查委员会批准了初始协议,其平均(SD)为59(21)天,并为其他站点添加了平均(SD )提交后的22(18)天。从中央机构审核委员会批准到首次启动站点的中位时间为47.5天(平均102.1;范围为1-282),从首次启动启动到首次参与者同意的中位时间为27天(平均37.5;范围为0-96)。 。从收到资金开始,数据库就绪的平均时间为3.5个月(平均4.0;范围为0-8)。在完成的4项研究中,所有站点的入学率均达到或超过预期,总体数据准确性为96%。
结论与相关性 NeuroNEXT启动了8项研究,成功地或提前完成了研究的入选,收集了高质量的数据,在高影响力的期刊上发表了主要结果,并为数名新的研究者提供了指导,专家统计和试验管理支持。在政府,学术界,行业,基金会和患者倡导团体之间成功建立了合作关系。临床试验协会可以有效地成功解决一系列重要的神经学研究和治疗问题。


























 京公网安备 11010802027423号
京公网安备 11010802027423号