当前位置:
X-MOL 学术
›
Anal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Electrochemically Lighting Up Luminophores at Similar Low Triggering Potentials with Mechanistic Insights.
Analytical Chemistry ( IF 7.4 ) Pub Date : 2020-04-01 , DOI: 10.1021/acs.analchem.0c00819 Li Fu 1 , Bin Zhang 1 , Kena Fu 1 , Xuwen Gao 1 , Guizheng Zou 1
Analytical Chemistry ( IF 7.4 ) Pub Date : 2020-04-01 , DOI: 10.1021/acs.analchem.0c00819 Li Fu 1 , Bin Zhang 1 , Kena Fu 1 , Xuwen Gao 1 , Guizheng Zou 1
Affiliation
|
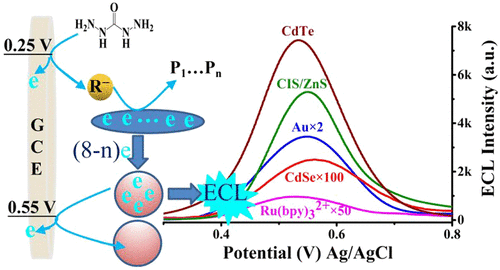
Electrochemiluminescence (ECL) with high electrode compatibility and less electrochemical interference has conventionally been envisioned by lowering the oxidative potential of luminophores and/or screening luminophores with a low oxidative potential. Herein, an alternative was developed by employing the environmental-friendly carbohydrazide as a coreactant, which enabled serial luminophores with oxidative-reduction ECL at one similar low triggering potential around 0.55 V versus Ag/AgCl, including Ru(bpy)32+ as well as CdTe, CdSe, CuInS2/ZnS, and Au nanocrystals. Because the eight-electron releasing process of carbohydrazide was electrochemically triggered at ∼0.25 V versus Ag/AgCl, the radicals generated via electrochemical oxidation of carbohydrazide could reduce the luminophores at a much lower potential than those of traditional coreactants. All the luminophore/carbohydrazide systems exhibited one ECL process around 0.55 V, which was about 0.65 V lower than that of a traditional Ru(bpy)32+/tri-n-propylamine system (typically around +1.2 V), and even lower than the oxidative potential of some luminophores. The ECL of the luminophore/carbohydrazide system was spectrally close to that of the corresponding luminophore/tri-n-propylamine system; the maximum emission wavelength of the low triggering potential ECL could shift from 540 to 783 nm via the selection of luminophores in this case. The coreactant screening strategy would be a favorable addition to the expected luminophore screening strategy for achieving enhanced ECL performance. This work created an avenue toward a deeper understanding of the ECL mechanism.
中文翻译:

借助机械原理,以相似的低触发电位对发光体进行电化学照明。
通常,通过降低发光体的氧化电势和/或筛选具有低氧化电势的发光体,可以预见具有高电极相容性和较少电化学干扰的电化学发光(ECL)。本文中,通过使用环保的碳酰肼作为共反应剂,开发了一种替代方案,该方案使具有氧化还原ECL的系列发光体在相对于Ag / AgCl的0.55 V附近具有相似的低触发电势,包括Ru(bpy)32+以及CdTe,CdSe,CuInS2 / ZnS和Au纳米晶体。因为相对于Ag / AgCl,碳酰肼的八电子释放过程是在〜0.25 V时被电化学触发的,所以通过碳酰肼的电化学氧化产生的自由基可以以比传统共反应物低得多的电势还原发光体。所有的发光体/碳酰肼系统在0.55 V左右都表现出一种ECL过程,这比传统的Ru(bpy)32 + / tri-n-丙胺系统(通常约为+1.2 V)低约0.65V。一些发光体的氧化电位。发光体/碳酰肼体系的ECL在光谱上接近于相应的发光体/三正丙胺体系的ECL;在这种情况下,通过选择发光体,低触发电势ECL的最大发射波长可以从540 nm变至783 nm。共反应物筛选策略将是预期的发光体筛选策略的有利补充,以实现增强的ECL性能。这项工作为深入了解ECL机制创造了一条途径。
更新日期:2020-04-23
中文翻译:

借助机械原理,以相似的低触发电位对发光体进行电化学照明。
通常,通过降低发光体的氧化电势和/或筛选具有低氧化电势的发光体,可以预见具有高电极相容性和较少电化学干扰的电化学发光(ECL)。本文中,通过使用环保的碳酰肼作为共反应剂,开发了一种替代方案,该方案使具有氧化还原ECL的系列发光体在相对于Ag / AgCl的0.55 V附近具有相似的低触发电势,包括Ru(bpy)32+以及CdTe,CdSe,CuInS2 / ZnS和Au纳米晶体。因为相对于Ag / AgCl,碳酰肼的八电子释放过程是在〜0.25 V时被电化学触发的,所以通过碳酰肼的电化学氧化产生的自由基可以以比传统共反应物低得多的电势还原发光体。所有的发光体/碳酰肼系统在0.55 V左右都表现出一种ECL过程,这比传统的Ru(bpy)32 + / tri-n-丙胺系统(通常约为+1.2 V)低约0.65V。一些发光体的氧化电位。发光体/碳酰肼体系的ECL在光谱上接近于相应的发光体/三正丙胺体系的ECL;在这种情况下,通过选择发光体,低触发电势ECL的最大发射波长可以从540 nm变至783 nm。共反应物筛选策略将是预期的发光体筛选策略的有利补充,以实现增强的ECL性能。这项工作为深入了解ECL机制创造了一条途径。


























 京公网安备 11010802027423号
京公网安备 11010802027423号