当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Occurrence of Biased Conformations as Precursors of Assembly States in Fibril Elongation of Amyloid-β Fibril Variants: An In Silico Study
The Journal of Physical Chemistry B ( IF 3.3 ) Pub Date : 2020-03-31 , DOI: 10.1021/acs.jpcb.0c01360 Rafael B. Frigori 1 , Fernando L. Barroso da Silva 2 , Patrícia P. D. Carvalho 3 , Nelson A. Alves 3
The Journal of Physical Chemistry B ( IF 3.3 ) Pub Date : 2020-03-31 , DOI: 10.1021/acs.jpcb.0c01360 Rafael B. Frigori 1 , Fernando L. Barroso da Silva 2 , Patrícia P. D. Carvalho 3 , Nelson A. Alves 3
Affiliation
|
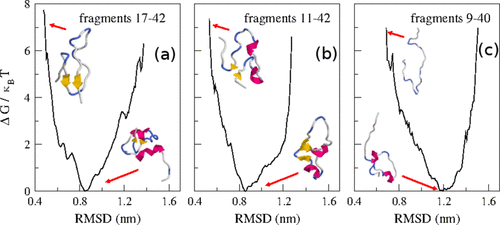
We investigate the prevalence, and so the role in the amyloidogenesis, of biased conformations in large ensembles of monomeric forms for Aβ42 and Aβ40 that can trigger the formation and growth of fibrils described by a dock–lock mechanism. We model such biased conformations as the structural monomeric units that constitute the Protein Data Bank fibrils 2beg, 2mxu, and 2lmn. These units were employed as templates to search for similar structures in statistical conformational ensembles of Aβ peptides generated by molecular dynamics with an accurate force field in explicit solvation, whose high quality is revealed by comparison with residual dipolar coupling (RDC) experiments. The conformational ensembles generated by these intrinsically disordered peptides do not contain conformations highly similar to the amyloidogenic templates. This is a consequence of the low thermodynamic stability exhibited by the template-like conformations. A further constant-pH Monte Carlo study has revealed that this stability can be increased by suitable pH conditions, which helps to trigger the fibril elongation. Moreover, our analyses on the free energy landscapes, hydrogen bond prevalences, and principal component analysis distributions emphasize the relevance of many-body long-range cooperative interactions, likely acting over the infrequent preexisting structurally biased conformations, to explain the fibrils’ emergence.
中文翻译:

淀粉样-β原纤维变体的原纤维伸长中作为装配态前体的偏向构象的发生:计算机模拟研究
我们研究了大范围单体形式的Aβ42和Aβ40的有偏差构象的流行及其在淀粉样蛋白生成中的作用,这些构象可以触发由船坞锁机制描述的原纤维的形成和生长。我们将这种偏向的构象建模为构成蛋白质数据库原纤维2beg,2mxu和2lmn的结构单体单元。这些单元被用作模板,以寻找由分子动力学产生的Aβ肽的统计构象集合中的相似结构,并在显式溶剂化中具有精确的力场,通过与残留偶极偶合(RDC)实验的比较揭示了其高质量。这些内在无序的肽产生的构象集合不包含与淀粉样蛋白生成模板高度相似的构象。这是模板样构象表现出的低热力学稳定性的结果。进一步的恒定pH值蒙特卡洛研究表明,可以通过合适的pH条件来增加这种稳定性,这有助于触发原纤维的伸长。此外,我们对自由能态,氢键流行度和主成分分析分布的分析强调了多体远程合作相互作用的相关性,可能是由于不常见的先前存在的结构偏倚构象,从而解释了原纤维的出现。
更新日期:2020-03-31
中文翻译:

淀粉样-β原纤维变体的原纤维伸长中作为装配态前体的偏向构象的发生:计算机模拟研究
我们研究了大范围单体形式的Aβ42和Aβ40的有偏差构象的流行及其在淀粉样蛋白生成中的作用,这些构象可以触发由船坞锁机制描述的原纤维的形成和生长。我们将这种偏向的构象建模为构成蛋白质数据库原纤维2beg,2mxu和2lmn的结构单体单元。这些单元被用作模板,以寻找由分子动力学产生的Aβ肽的统计构象集合中的相似结构,并在显式溶剂化中具有精确的力场,通过与残留偶极偶合(RDC)实验的比较揭示了其高质量。这些内在无序的肽产生的构象集合不包含与淀粉样蛋白生成模板高度相似的构象。这是模板样构象表现出的低热力学稳定性的结果。进一步的恒定pH值蒙特卡洛研究表明,可以通过合适的pH条件来增加这种稳定性,这有助于触发原纤维的伸长。此外,我们对自由能态,氢键流行度和主成分分析分布的分析强调了多体远程合作相互作用的相关性,可能是由于不常见的先前存在的结构偏倚构象,从而解释了原纤维的出现。


























 京公网安备 11010802027423号
京公网安备 11010802027423号