当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Comprehensive Characterization of Lipid-Guided G Protein-Coupled Receptor Dimerization
The Journal of Physical Chemistry B ( IF 3.3 ) Pub Date : 2020-03-31 , DOI: 10.1021/acs.jpcb.0c00062 Stefan Gahbauer 1 , Rainer A. Böckmann 1
The Journal of Physical Chemistry B ( IF 3.3 ) Pub Date : 2020-03-31 , DOI: 10.1021/acs.jpcb.0c00062 Stefan Gahbauer 1 , Rainer A. Böckmann 1
Affiliation
|
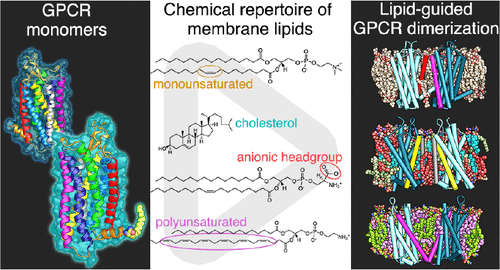
Dimerization of G protein-coupled receptors (GPCRs) is considered to take part in regulating the highly dynamic nature of receptor function. Intensive research unraveled a large variety of different dimer configurations with potentially distinct activity profiles. Studies are complicated by the critical role of the membrane environment for receptor dimerization, and experimental deficiencies in modulating the same. Here we chose a molecular dynamics strategy to characterize the potential of the large chemical lipid repertoire to steer dimerization fingerprints of the neurotensin 1 receptor. Unfavorable hydrophobic mismatch results in excessive dimerization whereas particular lipid features, e.g., anionic headgroups, induce specific dimer interfaces via direct protein–lipid interactions. Polyunsaturated fatty acids attenuate compact dimer formation by facilitated adhesion to the protein transmembrane surface, and receptor lipidation-induced conformational changes confer modulated protein–lipid and protein–protein interactions. Our results highlight the striking role of the membrane environment on GPCR dimerization with potential functional consequences.
中文翻译:

脂质引导的G蛋白偶联受体二聚体的全面表征。
G蛋白偶联受体(GPCR)的二聚化被认为参与调节受体功能的高度动态性。深入的研究揭示了各种不同的二聚体构型,这些构型可能具有不同的活性。膜环境对于受体二聚化的关键作用以及调节该膜的实验缺陷使研究变得复杂。在这里,我们选择了分子动力学策略来表征大型化学脂质库控制神经降压素1受体二聚化指纹的潜力。不利的疏水错配会导致过度的二聚化,而特定的脂质功能(例如阴离子头基)则通过直接的蛋白质-脂质相互作用诱导特定的二聚体界面。多不饱和脂肪酸通过促进与蛋白质跨膜表面的粘附而减弱了紧密的二聚体形成,并且受体脂化引起的构象变化赋予了调节的蛋白质-脂质和蛋白质-蛋白质相互作用。我们的结果强调了膜环境对GPCR二聚化的潜在作用,并可能产生功能性后果。
更新日期:2020-03-31
中文翻译:

脂质引导的G蛋白偶联受体二聚体的全面表征。
G蛋白偶联受体(GPCR)的二聚化被认为参与调节受体功能的高度动态性。深入的研究揭示了各种不同的二聚体构型,这些构型可能具有不同的活性。膜环境对于受体二聚化的关键作用以及调节该膜的实验缺陷使研究变得复杂。在这里,我们选择了分子动力学策略来表征大型化学脂质库控制神经降压素1受体二聚化指纹的潜力。不利的疏水错配会导致过度的二聚化,而特定的脂质功能(例如阴离子头基)则通过直接的蛋白质-脂质相互作用诱导特定的二聚体界面。多不饱和脂肪酸通过促进与蛋白质跨膜表面的粘附而减弱了紧密的二聚体形成,并且受体脂化引起的构象变化赋予了调节的蛋白质-脂质和蛋白质-蛋白质相互作用。我们的结果强调了膜环境对GPCR二聚化的潜在作用,并可能产生功能性后果。


























 京公网安备 11010802027423号
京公网安备 11010802027423号