当前位置:
X-MOL 学术
›
ACS Pharmacol. Transl. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Distinguishing Pharmacokinetics of Marketed Nanomedicine Formulations Using a Stable Isotope Tracer Assay.
ACS Pharmacology & Translational Science Pub Date : 2020-03-13 , DOI: 10.1021/acsptsci.0c00011 Sarah L Skoczen 1 , Kelsie S Snapp 1 , Rachael M Crist 1 , Darby Kozak 2 , Xiaohui Jiang 2 , Hao Liu 2 , Stephan T Stern 1
ACS Pharmacology & Translational Science Pub Date : 2020-03-13 , DOI: 10.1021/acsptsci.0c00011 Sarah L Skoczen 1 , Kelsie S Snapp 1 , Rachael M Crist 1 , Darby Kozak 2 , Xiaohui Jiang 2 , Hao Liu 2 , Stephan T Stern 1
Affiliation
|
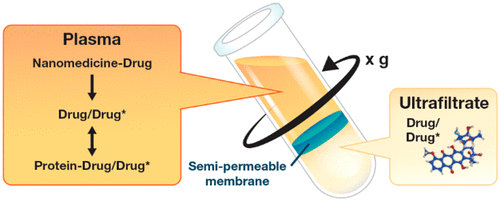
The pharmacokinetics of nanomedicines are complicated by the unique dispositional characteristics of the drug carrier. Most simplistically, the carrier could be a solubilizing platform that allows administration of a hydrophobic drug. Alternatively, the carrier could be stable and release the drug in a controlled manner, allowing for distribution of the carrier to influence distribution of the encapsulated drug. A third potential dispositional mechanism is carriers that are not stably complexed to the drug, but rather bind the drug in a dynamic equilibrium, similar to the binding of unbound drug to protein; since the nanocarrier has distributional and binding characteristics unlike plasma proteins, the equilibrium binding of drug to a nanocarrier can affect pharmacokinetics in unexpected ways, diverging from classical protein binding paradigms. The recently developed stable isotope tracer ultrafiltration assay (SITUA) for nanomedicine fractionation is uniquely suited for distinguishing and comparing these carrier/drug interactions. Here we present the the encapsulated, unencapsulated, and unbound drug fraction pharmacokinetic profiles in rats for marketed nanomedicines, representing examples of controlled release (doxorubicin liposomes, Doxil; and doxorubicin HCl liposome generic), equilibrium binding (paclitaxel cremophor micelle solution, Taxol generic), and solubilizing (paclitaxel albumin nanoparticle, Abraxane; and paclitaxel polylactic acid micelle, Genexol-PM) nanomedicine formulations. The utility of the SITUA method in differentiating these unique pharmacokinetic profiles and its potential for use in establishing generic nanomedicine bioequivalence are discussed.
中文翻译:

使用稳定的同位素示踪剂分析来区分市售纳米药物制剂的药代动力学。
纳米药物的药代动力学因药物载体的独特配置特性而变得复杂。最简单地,载体可以是允许施用疏水性药物的增溶平台。或者,载体可以是稳定的并且以受控的方式释放药物,从而允许载体的分布影响包封药物的分布。第三种潜在的处置机制是载体未稳定地与药物复合,而是以动态平衡结合药物,类似于未结合的药物与蛋白质的结合。由于纳米载体具有不同于血浆蛋白的分布和结合特性,因此药物与纳米载体的平衡结合会以意想不到的方式影响药代动力学,这与经典的蛋白质结合范例不同。最近开发的用于纳米药物分离的稳定同位素示踪剂超滤测定法(SITUA)特别适用于区分和比较这些载体/药物相互作用。在这里,我们介绍了市售纳米药物在大鼠中的胶囊化,未胶囊化和未结合的药物部分药代动力学概况,代表了控释(阿霉素脂质体,多西尔和阿霉素盐酸盐脂质体通用),平衡结合(紫杉醇克列莫弗胶束溶液,紫杉醇通用)的实例。 ,并增溶(紫杉醇白蛋白纳米颗粒,Abraxane;和紫杉醇聚乳酸胶束,Genexol-PM)纳米药物制剂。讨论了SITUA方法在区分这些独特的药代动力学特征方面的效用及其在建立通用纳米药物生物等效性中的潜力。
更新日期:2020-03-13
中文翻译:

使用稳定的同位素示踪剂分析来区分市售纳米药物制剂的药代动力学。
纳米药物的药代动力学因药物载体的独特配置特性而变得复杂。最简单地,载体可以是允许施用疏水性药物的增溶平台。或者,载体可以是稳定的并且以受控的方式释放药物,从而允许载体的分布影响包封药物的分布。第三种潜在的处置机制是载体未稳定地与药物复合,而是以动态平衡结合药物,类似于未结合的药物与蛋白质的结合。由于纳米载体具有不同于血浆蛋白的分布和结合特性,因此药物与纳米载体的平衡结合会以意想不到的方式影响药代动力学,这与经典的蛋白质结合范例不同。最近开发的用于纳米药物分离的稳定同位素示踪剂超滤测定法(SITUA)特别适用于区分和比较这些载体/药物相互作用。在这里,我们介绍了市售纳米药物在大鼠中的胶囊化,未胶囊化和未结合的药物部分药代动力学概况,代表了控释(阿霉素脂质体,多西尔和阿霉素盐酸盐脂质体通用),平衡结合(紫杉醇克列莫弗胶束溶液,紫杉醇通用)的实例。 ,并增溶(紫杉醇白蛋白纳米颗粒,Abraxane;和紫杉醇聚乳酸胶束,Genexol-PM)纳米药物制剂。讨论了SITUA方法在区分这些独特的药代动力学特征方面的效用及其在建立通用纳米药物生物等效性中的潜力。



























 京公网安备 11010802027423号
京公网安备 11010802027423号