当前位置:
X-MOL 学术
›
Eur. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Chemical Synthesis and Biological Effect on Xylem Formation of Xylemin and Its Analogues
European Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2020-04-16 , DOI: 10.1002/ejoc.202000322 Hiroyoshi Takamura 1 , Hiroyasu Motose 2 , Taichi Otsu 1 , Shiori Shinohara 2 , Ryugo Kouno 2 , Isao Kadota 1 , Taku Takahashi 2
European Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2020-04-16 , DOI: 10.1002/ejoc.202000322 Hiroyoshi Takamura 1 , Hiroyasu Motose 2 , Taichi Otsu 1 , Shiori Shinohara 2 , Ryugo Kouno 2 , Isao Kadota 1 , Taku Takahashi 2
Affiliation
|
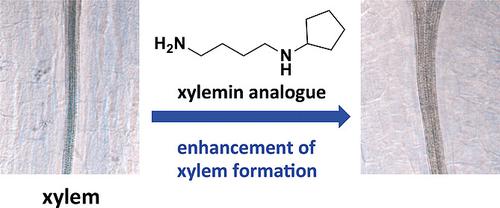
Xylemin (6 ) and its designed structural analogues 18 –23 , N ‐(4‐aminobutyl)alkylamines, were synthesized by 2‐nitrobenzenesulfonamide (Ns) strategy. Investigation of the improved synthesis of 20 –23 resulted in the development of one‐step synthesis of these analogues from the commercially available corresponding ketones. Biological assessment of the synthetic molecules elucidated that xylemin (6 ) and the analogue N ‐(4‐aminobutyl)cyclopentylamine (21 ) promoted the expression level of thermospermine synthase ACAULIS5 (ACL5 ) and enhanced xylem formation. In addition, xylemin (6 ) was found to significantly promote lateral root formation, whereas xylemin analogues 18 –23 including 21 did not. These results indicate that the analogue 21 has the potential as a novel inhibitor of thermospermine synthesis to work specifically in xylem differentiation.
中文翻译:

木质素及其类似物木质部形成的化学合成和生物效应
Xylemin (6) 及其设计的结构类似物 18-23,N-(4-氨基丁基) 烷基胺,通过 2-硝基苯磺酰胺 (Ns) 策略合成。对 20-23 的改进合成的研究导致从市售的相应酮一步合成这些类似物的发展。合成分子的生物学评估表明,木质素 (6) 和类似物 N-(4-氨基丁基) 环戊胺 (21) 促进了热精胺合酶 ACAULIS5 (ACL5) 的表达水平并增强了木质部的形成。此外,发现木质素 (6) 显着促进侧根形成,而木质素类似物 18 – 23 包括 21 则没有。
更新日期:2020-04-16
中文翻译:

木质素及其类似物木质部形成的化学合成和生物效应
Xylemin (6) 及其设计的结构类似物 18-23,N-(4-氨基丁基) 烷基胺,通过 2-硝基苯磺酰胺 (Ns) 策略合成。对 20-23 的改进合成的研究导致从市售的相应酮一步合成这些类似物的发展。合成分子的生物学评估表明,木质素 (6) 和类似物 N-(4-氨基丁基) 环戊胺 (21) 促进了热精胺合酶 ACAULIS5 (ACL5) 的表达水平并增强了木质部的形成。此外,发现木质素 (6) 显着促进侧根形成,而木质素类似物 18 – 23 包括 21 则没有。


























 京公网安备 11010802027423号
京公网安备 11010802027423号