当前位置:
X-MOL 学术
›
Environ. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Aluminosilicate-catalyzed electrochemical removal of ammonium cation from water -kinetics and selectivity.
Environmental Research ( IF 8.3 ) Pub Date : 2020-03-21 , DOI: 10.1016/j.envres.2020.109412 Ahmed Enmili 1 , Frédéric Monette 1 , Chakib Yahiat 1 , Makram Amor 1 , Ali Hedhli 2 , Abdelkrim Azzouz 3
Environmental Research ( IF 8.3 ) Pub Date : 2020-03-21 , DOI: 10.1016/j.envres.2020.109412 Ahmed Enmili 1 , Frédéric Monette 1 , Chakib Yahiat 1 , Makram Amor 1 , Ali Hedhli 2 , Abdelkrim Azzouz 3
Affiliation
|
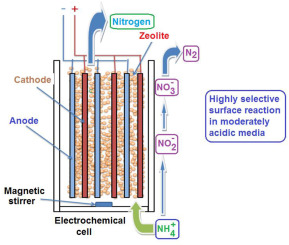
Aluminosilicate-catalyzed electrochemical decomposition of ammonium cation (NH4+) in water was investigated using NH4+-saturated clinoptilolite and copper-nickel electrodes in the presence of different salts and acidic species. The results showed beneficial roles of chloride anion and moderately acidic media. NH4+ adsorbed by the zeolites was converted with a 98% selectivity into nitrogen. The process was found to obey zero-order kinetics in the presence of clinoptilolite and a first order process when NaCl is added. Beneficial buffering effects of the zeolite and acidic species were registered. Clinoptilolite turned out to act as both catalyst and NH4+ reservoir. These results allow envisaging effective and waste-free technology in treating NH4+-rich aqueous effluents through total electroconversion into nitrogen using low cost aluminosilicates. Clay minerals, soils, sludges and natural water turbidity are potential catalysts for this purpose.
中文翻译:

铝硅酸盐催化的电化学从水中去除铵阳离子的动力学和选择性。
在不同盐和酸性物质存在下,使用NH4 +饱和斜发沸石和铜镍电极研究了铝硅酸盐催化的铵离子(NH4 +)在水中的电化学分解。结果显示出氯离子和中等酸性介质的有益作用。沸石吸附的NH4 +以98%的选择性转化为氮。发现该过程在斜发沸石存在下服从零级动力学,而在添加NaCl时服从一级过程。记录了沸石和酸性物质的有益缓冲作用。斜发沸石可同时充当催化剂和NH4 +储层。这些结果允许设想一种有效且无浪费的技术,通过使用低成本铝硅酸盐将总NH4 +转化为氮,可以处理富含NH4 +的废水。
更新日期:2020-03-22
中文翻译:

铝硅酸盐催化的电化学从水中去除铵阳离子的动力学和选择性。
在不同盐和酸性物质存在下,使用NH4 +饱和斜发沸石和铜镍电极研究了铝硅酸盐催化的铵离子(NH4 +)在水中的电化学分解。结果显示出氯离子和中等酸性介质的有益作用。沸石吸附的NH4 +以98%的选择性转化为氮。发现该过程在斜发沸石存在下服从零级动力学,而在添加NaCl时服从一级过程。记录了沸石和酸性物质的有益缓冲作用。斜发沸石可同时充当催化剂和NH4 +储层。这些结果允许设想一种有效且无浪费的技术,通过使用低成本铝硅酸盐将总NH4 +转化为氮,可以处理富含NH4 +的废水。



























 京公网安备 11010802027423号
京公网安备 11010802027423号