Tetrahedron ( IF 2.1 ) Pub Date : 2020-03-22 , DOI: 10.1016/j.tet.2020.131148 Kazunori Takahashi , Yuichi Arai , Mayumi Ikegami-Kawai , Toshio Honda
|
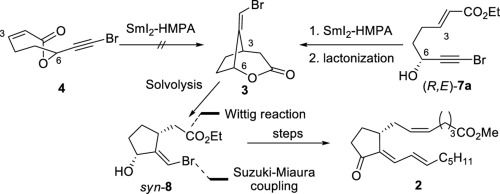
Prostaglandins (PGs) are recognized as a member of bioactive lipids. It is important to develop synthetic methods for further investigation of their biological activities. Among a variety of PGs, we decided to synthesize 15d-PGJ2 (1) by employing an SmI2-promoted radical cyclization as a key reaction. It was conceived that 3 would be assembled from bromolactone 4 utilizing the radical cyclization, however, 3 could not be obtained under the reaction conditions. On the other hand, the similar coupling reaction of bromoalkyne (R,E)-7a, the desired product syn-8 was given, via 3 for separation of diastereomers. The intermediate syn-8 was then transformed to Suh's intermediate (2) by several steps.
中文翻译:

的正式合成(+) - 15-脱氧Δ 12,14 -前列腺素Ĵ 2通过利用SMI 2 bromoalkynes和α,β -不饱和酯的促进的分子内偶合
前列腺素(PGs)被认为是生物活性脂质的成员。开发进一步研究其生物学活性的合成方法很重要。在各种PG中,我们决定通过使用SmI 2促进的自由基环化作为关键反应来合成15d-PGJ 2(1)。认为可以使用自由基环化作用从溴内酯4组装3,但是在反应条件下不能获得3。在另一方面,bromoalkyne的类似偶联反应([R ,ë) -图7a,所希望的产物顺式- 8通过3给出非对映异构体的分离。然后通过几个步骤将中间体syn - 8转化为Suh的中间体(2)。


























 京公网安备 11010802027423号
京公网安备 11010802027423号