当前位置:
X-MOL 学术
›
Chem. Eng. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Experiments and Correlations of the Solubility of γ‐DL‐Methionine in Binary Solvent Mixtures
Chemical Engineering & Technology ( IF 2.1 ) Pub Date : 2020-04-08 , DOI: 10.1002/ceat.201900642 Peetikamol Kongsamai 1 , Channakhone Phoumixay 2 , Lek Wantha 2
Chemical Engineering & Technology ( IF 2.1 ) Pub Date : 2020-04-08 , DOI: 10.1002/ceat.201900642 Peetikamol Kongsamai 1 , Channakhone Phoumixay 2 , Lek Wantha 2
Affiliation
|
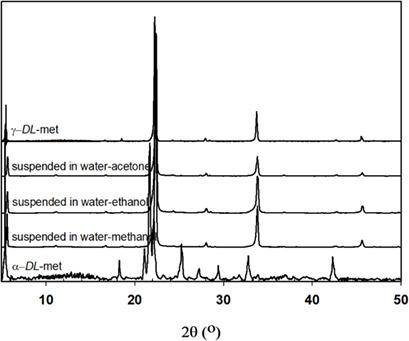
Antisolvents increase the supersaturation in the crystallization process which can enhance the product yield. The effect of an antisolvent on the solubility of γ‐DL‐methionine (γ‐DL‐met) in aqueous solution was investigated. The solubility of γ‐DL‐met was measured with various binary solvent mixtures. It improved with higher temperature but decreased with increasing the antisolvent mass fraction. Acetone showed the highest efficiency to reduce the solubility. The solubilities were correlated with the van't Hoff‐Jouyban‐Acree model and the modified Apelblat‐Jouyban‐Acree model. Both models fitted to the experimental results with high accuracy. Enthalpy, entropy, and Gibbs free energy of dissolution were determined by van't Hoff analysis. The thermodynamic properties indicated that the dissolution process is endothermic and entropy‐driven.
中文翻译:

γ-DL-蛋氨酸在二元溶剂混合物中溶解度的实验及相关性
抗溶剂会增加结晶过程中的过饱和度,从而可以提高产品收率。抗溶剂对的溶解度的影响γ-DL -甲硫氨酸(γ-DL -Met)在水溶液中进行了研究。γ‐DL的溶解度met是用各种二元溶剂混合物测量的。随着温度的升高其改善,但随着抗溶剂质量分数的增加而降低。丙酮显示出降低溶解度的最高效率。溶解度与van't Hoff-Jouyban-Acree模型和改良的Apelblat-Jouyban-Acree模型相关。两种模型都非常符合实验结果。焓,熵和吉布斯分解自由能通过van't Hoff分析确定。热力学性质表明溶解过程是吸热的和熵驱动的。
更新日期:2020-04-08
中文翻译:

γ-DL-蛋氨酸在二元溶剂混合物中溶解度的实验及相关性
抗溶剂会增加结晶过程中的过饱和度,从而可以提高产品收率。抗溶剂对的溶解度的影响γ-DL -甲硫氨酸(γ-DL -Met)在水溶液中进行了研究。γ‐DL的溶解度met是用各种二元溶剂混合物测量的。随着温度的升高其改善,但随着抗溶剂质量分数的增加而降低。丙酮显示出降低溶解度的最高效率。溶解度与van't Hoff-Jouyban-Acree模型和改良的Apelblat-Jouyban-Acree模型相关。两种模型都非常符合实验结果。焓,熵和吉布斯分解自由能通过van't Hoff分析确定。热力学性质表明溶解过程是吸热的和熵驱动的。


























 京公网安备 11010802027423号
京公网安备 11010802027423号