当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Engineering a bacterial sialyltransferase for di-sialylation of a therapeutic antibody
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2020-03-20 , DOI: 10.1039/d0ob00276c Mingqun Wang 1, 2, 3, 4, 5 , Yue Wang 1, 2, 3, 4, 5 , Kaimeng Liu 1, 2, 3, 4, 5 , Xiaodong Dou 1, 2, 3, 4, 5 , Zhenming Liu 1, 2, 3, 4, 5 , Liangren Zhang 1, 2, 3, 4, 5 , Xin-Shan Ye 1, 2, 3, 4, 5
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2020-03-20 , DOI: 10.1039/d0ob00276c Mingqun Wang 1, 2, 3, 4, 5 , Yue Wang 1, 2, 3, 4, 5 , Kaimeng Liu 1, 2, 3, 4, 5 , Xiaodong Dou 1, 2, 3, 4, 5 , Zhenming Liu 1, 2, 3, 4, 5 , Liangren Zhang 1, 2, 3, 4, 5 , Xin-Shan Ye 1, 2, 3, 4, 5
Affiliation
|
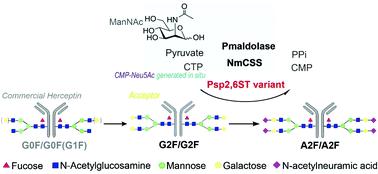
Terminal α-2,6-sialylation of N-glycans is a humanized glycosylation that affects the properties and efficacy of therapeutic glycoproteins. Fc di-sialylation (a biantennary N-glycan with two α-2,6-linked sialic acids) of IgG antibodies imparts them with enhanced anti-inflammatory activity and other roles. However, the microheterogeneity of N-glycoforms presents a challenge for therapeutic development. Therefore, controlled sialylation has drawn considerable attention, but direct access to well-defined di-sialylated antibodies remains limited. Herein, a one-pot three-enzyme protocol was developed by engineering a bacterial sialyltransferase to facilitate the modification of therapeutic antibodies with N-acetylneuraminic acid or its derivatives towards optimized glycosylation. To overcome the low proficiency of bacterial sialyltransferase in antibody remodeling, the Photobacterium sp. JT-ISH-224 α-2,6-sialyltransferase (Psp2,6ST) was genetically engineered by terminal truncation and site-directed mutagenesis based on its protein crystal structure. With the optimized reaction conditions and using activity-based screening of various Psp2,6ST variants, a truncated mutant Psp2,6ST (111–511)-His6 A235M/A366G was shown to effectively improve the catalytic efficiency of antibody di-sialylation. Herceptin and the donor substrate promiscuity allow the introduction of bioorthogonal modifications of N-acetylneuraminic acid into antibodies for site-specific conjugation. 2-AB hydrophilic interaction chromatography analysis of the released N-glycans and intact mass characterization confirmed the high di-sialylation of Herceptin via the optimized one-pot three-enzyme reaction. This study established a versatile enzymatic approach for producing highly di-sialylated IgG antibodies. It provides new insights into engineering bacterial sialyltransferase for adaptation to the enzymatic glycoengineering of therapeutic antibodies and the glycosite-specific conjugation of antibodies.
中文翻译:

工程化细菌唾液酸转移酶以进行治疗性抗体的二唾液酸化
N-聚糖的末端α-2,6-唾液酸化是人源化的糖基化,其影响治疗性糖蛋白的性质和功效。IgG抗体的Fc双唾液酸化作用(双天线N-聚糖带有两个由α-2,6-连接的唾液酸)可增强抗炎活性和其他作用。然而,N-糖型的微异质性为治疗发展提出了挑战。因此,受控的唾液酸化引起了相当大的关注,但是直接获得定义明确的二唾液酸化抗体仍然受到限制。在本文中,通过工程改造细菌唾液酸转移酶来开发一锅三酶方案,以促进用N修饰治疗性抗体-乙酰神经氨酸或其衍生物可优化糖基化。为了克服细菌唾液酸转移酶在抗体重塑中的低水平,Photobacter sp。JT-ISH- 224α-2,6-唾液酸转移酶(Psp2,6ST)通过末端截短和基于其蛋白质晶体结构的定点诱变进行基因工程设计。通过优化的反应条件并使用基于活性的各种Psp2,6ST变体筛选,截短的突变体Psp2,6ST(111-511)-His 6 A235M / A366G被证明可有效提高抗体二唾液酸化的催化效率。赫赛汀和供体底物的混杂使N的生物正交修饰得以引入-乙酰神经氨酸转化为抗体,用于位点特异性缀合。释放的N-聚糖的2-AB亲水相互作用色谱分析和完整的质量表征证实,通过优化的一锅三酶反应,赫赛汀具有很高的双唾液酸化作用。这项研究建立了一种通用的酶促方法来生产高度二唾液酸化的IgG抗体。它为改造细菌唾液酸转移酶提供了新的见识,以适应治疗性抗体的酶促糖工程化和抗体的糖位特异性缀合。
更新日期:2020-04-24
中文翻译:

工程化细菌唾液酸转移酶以进行治疗性抗体的二唾液酸化
N-聚糖的末端α-2,6-唾液酸化是人源化的糖基化,其影响治疗性糖蛋白的性质和功效。IgG抗体的Fc双唾液酸化作用(双天线N-聚糖带有两个由α-2,6-连接的唾液酸)可增强抗炎活性和其他作用。然而,N-糖型的微异质性为治疗发展提出了挑战。因此,受控的唾液酸化引起了相当大的关注,但是直接获得定义明确的二唾液酸化抗体仍然受到限制。在本文中,通过工程改造细菌唾液酸转移酶来开发一锅三酶方案,以促进用N修饰治疗性抗体-乙酰神经氨酸或其衍生物可优化糖基化。为了克服细菌唾液酸转移酶在抗体重塑中的低水平,Photobacter sp。JT-ISH- 224α-2,6-唾液酸转移酶(Psp2,6ST)通过末端截短和基于其蛋白质晶体结构的定点诱变进行基因工程设计。通过优化的反应条件并使用基于活性的各种Psp2,6ST变体筛选,截短的突变体Psp2,6ST(111-511)-His 6 A235M / A366G被证明可有效提高抗体二唾液酸化的催化效率。赫赛汀和供体底物的混杂使N的生物正交修饰得以引入-乙酰神经氨酸转化为抗体,用于位点特异性缀合。释放的N-聚糖的2-AB亲水相互作用色谱分析和完整的质量表征证实,通过优化的一锅三酶反应,赫赛汀具有很高的双唾液酸化作用。这项研究建立了一种通用的酶促方法来生产高度二唾液酸化的IgG抗体。它为改造细菌唾液酸转移酶提供了新的见识,以适应治疗性抗体的酶促糖工程化和抗体的糖位特异性缀合。



























 京公网安备 11010802027423号
京公网安备 11010802027423号