当前位置:
X-MOL 学术
›
J. Mater. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Catalyzing zinc-ion intercalation in hydrated vanadates for aqueous zinc-ion batteries
Journal of Materials Chemistry A ( IF 11.9 ) Pub Date : 2020-03-20 , DOI: 10.1039/d0ta01468k Chaofeng Liu 1, 2, 3, 4 , Meng Tian 1, 2, 3, 4 , Mingshan Wang 1, 2, 3, 4 , Jiqi Zheng 1, 2, 3, 4 , Shuhua Wang 5, 6, 7, 8 , Mengyu Yan 1, 2, 3, 4 , Zhaojie Wang 1, 2, 3, 4 , Zhengmao Yin 8, 9, 10, 11 , Jihui Yang 1, 2, 3, 4 , Guozhong Cao 1, 2, 3, 4
Journal of Materials Chemistry A ( IF 11.9 ) Pub Date : 2020-03-20 , DOI: 10.1039/d0ta01468k Chaofeng Liu 1, 2, 3, 4 , Meng Tian 1, 2, 3, 4 , Mingshan Wang 1, 2, 3, 4 , Jiqi Zheng 1, 2, 3, 4 , Shuhua Wang 5, 6, 7, 8 , Mengyu Yan 1, 2, 3, 4 , Zhaojie Wang 1, 2, 3, 4 , Zhengmao Yin 8, 9, 10, 11 , Jihui Yang 1, 2, 3, 4 , Guozhong Cao 1, 2, 3, 4
Affiliation
|
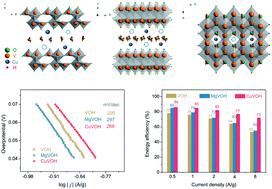
Hydrated vanadium pentoxide (VOH) can deliver a gravimetric capacity as high as 400 mA h g−1 owing to the variable valence states of the V cation from 5+ to 3+ in an aqueous zinc ion battery. The incorporation of divalent transition metal cations has been demonstrated to overcome the structural instability, sluggish kinetics, fast capacity degradation, and serious polarization. The current study reveals that the catalytic effects of transition metal cations are probably the key to the significantly improved electrochemical properties and battery performance because of the higher covalent character of 55% in the Cu–O bond in comparison with 32% in the Mg–O bond in the respective samples. Cu(II) pre-inserted VOH (CuVOH) possesses a significantly enhanced intercalation storage capacity, an increased discharge voltage, great transport properties, and reduced polarization, while both VOH and Mg(II) pre-inserted VOH (MgVOH) demonstrate similar electrochemical properties and performances, indicating that the incorporation of Mg cations has little or no impact. For example, CuVOH has a redox voltage gap of 0.02 V, much smaller than 0.25 V for VOH and 0.27 V for MgVOH. CuVOH shows an enhanced exchange current density of 0.23 A g−1, compared to 0.20 A g−1 for VOH and 0.19 A g−1 for MgVOH. CuVOH delivers a zinc ion storage capacity of 379 mA h g−1, higher than 349 mA h g−1 for MgVOH and 337 mA h g−1 for VOH at 0.5 A g−1. CuVOH shows an energy efficiency of 72%, superior to 53% for VOH and 55% for MgVOH. All of the results suggest that pre-inserted Cu(II) cations played a critical role in catalyzing the zinc ion intercalation reaction, while the Mg(II) cations did not exert a detectable catalytic effect.
中文翻译:

水性锌离子电池中水合钒酸盐中的锌离子嵌入催化
由于在水性锌离子电池中V阳离子的价态从5+变为3+,水合五氧化二钒(VOH)的重量分析能力高达400 mA hg -1。已证明掺入二价过渡金属阳离子可克服结构不稳定性,反应迟钝的动力学,快速的容量降解和严重的极化。当前的研究表明,过渡金属阳离子的催化作用可能是显着改善电化学性能和电池性能的关键,因为Cu-O键中55%的共价特性较高,而Mg-O中为32%的更高共价特性。在各个样品中键合。铜(II)预插入的VOH(CuVOH)具有显着增强的插层存储容量,增加的放电电压,出色的传输性能和降低的极化性能,而VOH和Mg(II)的预插入VOH(MgVOH)均具有相似的电化学性能,表明掺入镁阳离子几乎没有影响。例如,CuVOH的氧化还原电压间隙为0.02 V,远小于VOH的0.25 V和MgVOH的0.27V。与VOH的0.20 A g -1和MgVOH的0.19 A g -1相比,CuVOH的交换电流密度提高了0.23 A g -1。CuVOH的锌离子存储容量为379 mA hg -1,高于349 mA hg -1在0.5 A g -1下的MOH和337 mA hg -1的VOH 。CuVOH的能效为72%,优于VOH的53%和MgVOH的55%。所有结果表明,预先插入的Cu(II)阳离子在催化锌离子嵌入反应中起关键作用,而Mg(II)阳离子则没有发挥可检测的催化作用。
更新日期:2020-03-20
中文翻译:

水性锌离子电池中水合钒酸盐中的锌离子嵌入催化
由于在水性锌离子电池中V阳离子的价态从5+变为3+,水合五氧化二钒(VOH)的重量分析能力高达400 mA hg -1。已证明掺入二价过渡金属阳离子可克服结构不稳定性,反应迟钝的动力学,快速的容量降解和严重的极化。当前的研究表明,过渡金属阳离子的催化作用可能是显着改善电化学性能和电池性能的关键,因为Cu-O键中55%的共价特性较高,而Mg-O中为32%的更高共价特性。在各个样品中键合。铜(II)预插入的VOH(CuVOH)具有显着增强的插层存储容量,增加的放电电压,出色的传输性能和降低的极化性能,而VOH和Mg(II)的预插入VOH(MgVOH)均具有相似的电化学性能,表明掺入镁阳离子几乎没有影响。例如,CuVOH的氧化还原电压间隙为0.02 V,远小于VOH的0.25 V和MgVOH的0.27V。与VOH的0.20 A g -1和MgVOH的0.19 A g -1相比,CuVOH的交换电流密度提高了0.23 A g -1。CuVOH的锌离子存储容量为379 mA hg -1,高于349 mA hg -1在0.5 A g -1下的MOH和337 mA hg -1的VOH 。CuVOH的能效为72%,优于VOH的53%和MgVOH的55%。所有结果表明,预先插入的Cu(II)阳离子在催化锌离子嵌入反应中起关键作用,而Mg(II)阳离子则没有发挥可检测的催化作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号