当前位置:
X-MOL 学术
›
PLOS Negl. Trop. Dis.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Efficacy of single versus four repeated doses of praziquantel against Schistosoma mansoni infection in school-aged children from Côte d'Ivoire based on Kato-Katz and POC-CCA: An open-label, randomised controlled trial (RePST).
PLOS Neglected Tropical Diseases ( IF 3.8 ) Pub Date : 2020-03-20 , DOI: 10.1371/journal.pntd.0008189 Pytsje T Hoekstra 1 , Miriam Casacuberta-Partal 1 , Lisette van Lieshout 1 , Paul L A M Corstjens 2 , Roula Tsonaka 3 , Rufin K Assaré 4, 5, 6, 7 , Kigbafori D Silué 4, 5 , Aboulaye Meité 8 , Eliézer K N'Goran 4, 5 , Yves K N'Gbesso 9 , Abena S Amoah 1, 10, 11 , Meta Roestenberg 1, 12 , Stefanie Knopp 6, 7 , Jürg Utzinger 6, 7 , Jean T Coulibaly 4, 5, 6, 7 , Govert J van Dam 1
PLOS Neglected Tropical Diseases ( IF 3.8 ) Pub Date : 2020-03-20 , DOI: 10.1371/journal.pntd.0008189 Pytsje T Hoekstra 1 , Miriam Casacuberta-Partal 1 , Lisette van Lieshout 1 , Paul L A M Corstjens 2 , Roula Tsonaka 3 , Rufin K Assaré 4, 5, 6, 7 , Kigbafori D Silué 4, 5 , Aboulaye Meité 8 , Eliézer K N'Goran 4, 5 , Yves K N'Gbesso 9 , Abena S Amoah 1, 10, 11 , Meta Roestenberg 1, 12 , Stefanie Knopp 6, 7 , Jürg Utzinger 6, 7 , Jean T Coulibaly 4, 5, 6, 7 , Govert J van Dam 1
Affiliation
|
BACKGROUND
Preventive chemotherapy with praziquantel (PZQ) is the cornerstone of schistosomiasis control. However, a single dose of PZQ (40 mg/kg) does not cure all infections. Repeated doses of PZQ at short intervals might increase efficacy in terms of cure rate (CR) and intensity reduction rate (IRR). Here, we determined the efficacy of a single versus four repeated treatments with PZQ on Schistosoma mansoni infection in school-aged children from Côte d'Ivoire, using two different diagnostic tests.
METHODS
An open-label, randomized controlled trial was conducted from October 2018 to January 2019. School-aged children with a confirmed S. mansoni infection based on Kato-Katz (KK) and point-of-care circulating cathodic antigen (POC-CCA) urine cassette test were randomly assigned to receive either a single or four repeated doses of PZQ, administered at two-week intervals. The primary outcome was the difference in CR between the two treatment arms, measured by triplicate KK thick smears 10 weeks after the first treatment. Secondary outcomes included CR estimated by POC-CCA, IRR by KK and POC-CCA, and safety of repeated PZQ administration.
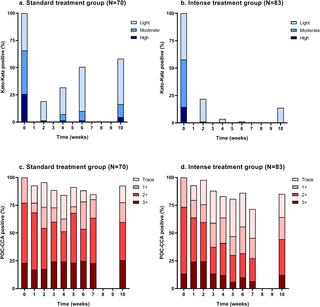
PRINCIPAL FINDINGS
During baseline screening, 1,022 children were assessed for eligibility of whom 153 (15%) had a detectable S. mansoni infection, and hence, were randomized to the standard treatment group (N = 70) and the intense treatment group (N = 83). Based on KK, the CR was 42% (95% confidence interval (CI) 31-52%) in the standard treatment group and 86% (95% CI 75-92%) in the intense treatment group. Observed IRR was 72% (95% CI 55-83%) in the standard treatment group and 95% (95% CI 85-98%) in the intense treatment group. The CR estimated by POC-CCA was 18% (95% CI 11-27%) and 36% (95% CI 26-46%) in the standard and intense treatment group, respectively. Repeated PZQ treatment did not result in a higher number of adverse events.
CONCLUSION/SIGNIFICANCE
The observed CR using KK was significantly higher after four repeated treatments compared to a single treatment, without an increase in adverse events. Using POC-CCA, the observed CR was significantly lower than measured by KK, indicating that PZQ may be considerably less efficacious as concluded by KK. Our findings highlight the need for reliable and more accurate diagnostic tools, which are essential for monitoring treatment efficacy, identifying changes in transmission, and accurately quantifying the intensity of infection in distinct populations. In addition, the higher CR in the intense treatment group suggests that more focused and intense PZQ treatment can help to advance schistosomiasis control.
TRIAL REGISTRATION
www.clinicaltrials.gov NCT02868385.
中文翻译:

根据Kato-Katz和POC-CCA,单次或多次重复剂量的吡喹酮对科特迪瓦学龄儿童的曼氏血吸虫感染的疗效:一项开放标签,随机对照试验(RePST)。
背景技术吡喹酮(PZQ)预防性化学疗法是控制血吸虫病的基石。但是,单剂量的PZQ(40 mg / kg)不能治愈所有感染。在短间隔内重复服用PZQ可能会提高治愈率(CR)和强度降低率(IRR)的功效。在这里,我们使用两种不同的诊断测试,确定了单次或多次重复使用PZQ治疗科特迪瓦学龄儿童曼氏血吸虫感染的效果。方法从2018年10月至2019年1月进行了一项开放标签,随机对照试验。根据Kato-Katz(KK)和即时护理循环阴极抗原(POC-CCA)确诊曼氏沙门氏菌感染的学龄儿童)尿液盒试验被随机分配为接受单剂量或四剂重复剂量的PZQ,每两周服用一次。主要结局是两个治疗组之间的CR差异,通过第一次治疗10周后一式三份KK厚涂片测量。次要结果包括通过POC-CCA评估的CR,通过KK和POC-CCA评估的IRR,以及重复施用PZQ的安全性。主要发现在基线筛查期间,评估了1,022名儿童的资格,其中153名(15%)有可检测的曼氏沙门氏菌感染,因此被随机分为标准治疗组(N = 70)和强化治疗组(N = 83)。基于KK,标准治疗组的CR为42%(95%置信区间(CI)31-52%),而强治疗组的CR为86%(95%CI 75-92%)。在标准治疗组中观察到的IRR为72%(95%CI 55-83%),在强治疗组中观察到的IRR为95%(95%CI 85-98%)。在标准治疗组和强化治疗组中,POC-CCA估计的CR分别为18%(95%CI 11-27%)和36%(95%CI 26-46%)。重复进行PZQ治疗不会导致更多的不良事件。结论/意义与单独治疗相比,经四次重复治疗后观察到的使用KK的CR显着更高,且无不良事件增加。使用POC-CCA,观察到的CR显着低于KK测得的CR,表明PZQ的功效可能远低于KK得出的结论。我们的发现凸显了对可靠且更准确的诊断工具的需求,这些工具对于监控治疗效果,识别传播变化以及准确量化不同人群的感染强度至关重要。此外,强化治疗组中较高的CR表明,更集中和强化的PZQ治疗可以帮助促进血吸虫病的控制。试用注册www.clinicaltrials.gov NCT02868385。
更新日期:2020-03-21
中文翻译:

根据Kato-Katz和POC-CCA,单次或多次重复剂量的吡喹酮对科特迪瓦学龄儿童的曼氏血吸虫感染的疗效:一项开放标签,随机对照试验(RePST)。
背景技术吡喹酮(PZQ)预防性化学疗法是控制血吸虫病的基石。但是,单剂量的PZQ(40 mg / kg)不能治愈所有感染。在短间隔内重复服用PZQ可能会提高治愈率(CR)和强度降低率(IRR)的功效。在这里,我们使用两种不同的诊断测试,确定了单次或多次重复使用PZQ治疗科特迪瓦学龄儿童曼氏血吸虫感染的效果。方法从2018年10月至2019年1月进行了一项开放标签,随机对照试验。根据Kato-Katz(KK)和即时护理循环阴极抗原(POC-CCA)确诊曼氏沙门氏菌感染的学龄儿童)尿液盒试验被随机分配为接受单剂量或四剂重复剂量的PZQ,每两周服用一次。主要结局是两个治疗组之间的CR差异,通过第一次治疗10周后一式三份KK厚涂片测量。次要结果包括通过POC-CCA评估的CR,通过KK和POC-CCA评估的IRR,以及重复施用PZQ的安全性。主要发现在基线筛查期间,评估了1,022名儿童的资格,其中153名(15%)有可检测的曼氏沙门氏菌感染,因此被随机分为标准治疗组(N = 70)和强化治疗组(N = 83)。基于KK,标准治疗组的CR为42%(95%置信区间(CI)31-52%),而强治疗组的CR为86%(95%CI 75-92%)。在标准治疗组中观察到的IRR为72%(95%CI 55-83%),在强治疗组中观察到的IRR为95%(95%CI 85-98%)。在标准治疗组和强化治疗组中,POC-CCA估计的CR分别为18%(95%CI 11-27%)和36%(95%CI 26-46%)。重复进行PZQ治疗不会导致更多的不良事件。结论/意义与单独治疗相比,经四次重复治疗后观察到的使用KK的CR显着更高,且无不良事件增加。使用POC-CCA,观察到的CR显着低于KK测得的CR,表明PZQ的功效可能远低于KK得出的结论。我们的发现凸显了对可靠且更准确的诊断工具的需求,这些工具对于监控治疗效果,识别传播变化以及准确量化不同人群的感染强度至关重要。此外,强化治疗组中较高的CR表明,更集中和强化的PZQ治疗可以帮助促进血吸虫病的控制。试用注册www.clinicaltrials.gov NCT02868385。



























 京公网安备 11010802027423号
京公网安备 11010802027423号