Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Efficacy of levetiracetam, fosphenytoin, and valproate for established status epilepticus by age group (ESETT): a double-blind, responsive-adaptive, randomised controlled trial.
The Lancet ( IF 168.9 ) Pub Date : 2020-03-20 , DOI: 10.1016/s0140-6736(20)30611-5 James M Chamberlain 1 , Jaideep Kapur 2 , Shlomo Shinnar 3 , Jordan Elm 4 , Maija Holsti 5 , Lynn Babcock 6 , Alex Rogers 7 , William Barsan 8 , James Cloyd 9 , Daniel Lowenstein 10 , Thomas P Bleck 11 , Robin Conwit 12 , Caitlyn Meinzer 4 , Hannah Cock 13 , Nathan B Fountain 2 , Ellen Underwood 4 , Jason T Connor 14 , Robert Silbergleit 8 , ,
The Lancet ( IF 168.9 ) Pub Date : 2020-03-20 , DOI: 10.1016/s0140-6736(20)30611-5 James M Chamberlain 1 , Jaideep Kapur 2 , Shlomo Shinnar 3 , Jordan Elm 4 , Maija Holsti 5 , Lynn Babcock 6 , Alex Rogers 7 , William Barsan 8 , James Cloyd 9 , Daniel Lowenstein 10 , Thomas P Bleck 11 , Robin Conwit 12 , Caitlyn Meinzer 4 , Hannah Cock 13 , Nathan B Fountain 2 , Ellen Underwood 4 , Jason T Connor 14 , Robert Silbergleit 8 , ,
Affiliation
|
BACKGROUND
Benzodiazepine-refractory, or established, status epilepticus is thought to be of similar pathophysiology in children and adults, but differences in underlying aetiology and pharmacodynamics might differentially affect response to therapy. In the Established Status Epilepticus Treatment Trial (ESETT) we compared the efficacy and safety of levetiracetam, fosphenytoin, and valproate in established status epilepticus, and here we describe our results after extending enrolment in children to compare outcomes in three age groups.
METHODS
In this multicentre, double-blind, response-adaptive, randomised controlled trial, we recruited patients from 58 hospital emergency departments across the USA. Patients were eligible for inclusion if they were aged 2 years or older, had been treated for a generalised convulsive seizure of longer than 5 min duration with adequate doses of benzodiazepines, and continued to have persistent or recurrent convulsions in the emergency department for at least 5 min and no more than 30 min after the last dose of benzodiazepine. Patients were randomly assigned in a response-adaptive manner, using Bayesian methods and stratified by age group (<18 years, 18-65 years, and >65 years), to levetiracetam, fosphenytoin, or valproate. All patients, investigators, study staff, and pharmacists were masked to treatment allocation. The primary outcome was absence of clinically apparent seizures with improved consciousness and without additional antiseizure medication at 1 h from start of drug infusion. The primary safety outcome was life-threatening hypotension or cardiac arrhythmia. The efficacy and safety outcomes were analysed by intention to treat. This study is registered in ClinicalTrials.gov, NCT01960075.
FINDINGS
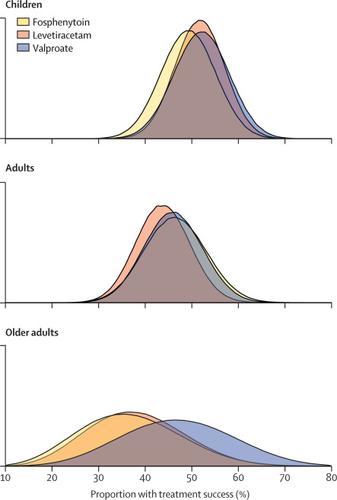
Between Nov 3, 2015, and Dec 29, 2018, we enrolled 478 patients and 462 unique patients were included: 225 children (aged <18 years), 186 adults (18-65 years), and 51 older adults (>65 years). 175 (38%) patients were randomly assigned to levetiracetam, 142 (31%) to fosphenyltoin, and 145 (31%) were to valproate. Baseline characteristics were balanced across treatments within age groups. The primary efficacy outcome was met in those treated with levetiracetam for 52% (95% credible interval 41-62) of children, 44% (33-55) of adults, and 37% (19-59) of older adults; with fosphenytoin in 49% (38-61) of children, 46% (34-59) of adults, and 35% (17-59) of older adults; and with valproate in 52% (41-63) of children, 46% (34-58) of adults, and 47% (25-70) of older adults. No differences were detected in efficacy or primary safety outcome by drug within each age group. With the exception of endotracheal intubation in children, secondary safety outcomes did not significantly differ by drug within each age group.
INTERPRETATION
Children, adults, and older adults with established status epilepticus respond similarly to levetiracetam, fosphenytoin, and valproate, with treatment success in approximately half of patients. Any of the three drugs can be considered as a potential first-choice, second-line drug for benzodiazepine-refractory status epilepticus.
FUNDING
National Institute of Neurological Disorders and Stroke, National Institutes of Health.
中文翻译:

左乙拉西坦、磷苯妥英和丙戊酸钠对按年龄组确定的癫痫持续状态 (ESETT) 的疗效:一项双盲、反应适应性随机对照试验。
背景苯二氮卓类药物难治性或确定的癫痫持续状态被认为在儿童和成人中具有相似的病理生理学,但潜在病因学和药效学的差异可能会不同地影响对治疗的反应。在确定的癫痫持续状态治疗试验 (ESETT) 中,我们比较了左乙拉西坦、磷苯妥英和丙戊酸盐在确定的癫痫持续状态中的疗效和安全性,在这里我们描述了我们在扩大儿童入组后的结果,以比较三个年龄组的结果。方法 在这项多中心、双盲、响应适应性随机对照试验中,我们从全美 58 家医院急诊科招募了患者。如果患者年龄在 2 岁或以上,则他们符合入选条件,已接受足量苯二氮卓类持续时间超过 5 分钟的全身性惊厥发作治疗,并且在最后一次苯二氮卓类药物给药后至少 5 分钟且不超过 30 分钟内在急诊室继续出现持续或反复发作的惊厥。使用贝叶斯方法并按年龄组(<18 岁、18-65 岁和>65 岁)分层,患者以反应适应性方式被随机分配至左乙拉西坦、磷苯妥英或丙戊酸盐。所有患者、研究人员、研究人员和药剂师均对治疗分配设盲。主要结果是在药物输注开始后 1 小时内没有临床明显的癫痫发作,意识得到改善,并且没有额外的抗癫痫药物。主要安全性结果是危及生命的低血压或心律失常。通过意向治疗分析疗效和安全性结果。本研究在 ClinicalTrials.gov 注册,NCT01960075。2015 年 11 月 3 日至 2018 年 12 月 29 日期间,我们招募了 478 名患者,其中包括 462 名独特患者:225 名儿童(年龄 <18 岁)、186 名成人(18-65 岁)和 51 名老年人(>65 岁) ). 175 名 (38%) 患者被随机分配至左乙拉西坦组,142 名 (31%) 至磷苯妥英组,145 名 (31%) 至丙戊酸盐组。基线特征在不同年龄组内的治疗之间是平衡的。在接受左乙拉西坦治疗的儿童中,52%(95% 可信区间 41-62)、44%(33-55)的成人和 37%(19-59)的老年人达到了主要疗效结果;49% (38-61) 的儿童、46% (34-59) 的成人和 35% (17-59) 的老年人使用磷苯妥英;52% (41-63) 的儿童、46% (34-58) 的成年人和 47% (25-70) 的老年人使用丙戊酸盐。在每个年龄组中,药物的疗效或主要安全性结果没有发现差异。除儿童气管插管外,次要安全性结果在每个年龄组中因药物而异。解释 确定性癫痫持续状态的儿童、成人和老年人对左乙拉西坦、磷苯妥英和丙戊酸钠的反应相似,大约一半的患者治疗成功。这三种药物中的任何一种都可以被认为是苯二氮卓类药物难治性癫痫持续状态的潜在首选、二线药物。资助国家神经疾病和中风研究所,美国国立卫生研究院。在每个年龄组中,药物的疗效或主要安全性结果没有发现差异。除儿童气管插管外,次要安全性结果在每个年龄组中因药物而异。解释 确定性癫痫持续状态的儿童、成人和老年人对左乙拉西坦、磷苯妥英和丙戊酸钠的反应相似,大约一半的患者治疗成功。这三种药物中的任何一种都可以被认为是苯二氮卓类药物难治性癫痫持续状态的潜在首选、二线药物。资助国家神经疾病和中风研究所,美国国立卫生研究院。在每个年龄组中,药物的疗效或主要安全性结果没有发现差异。除儿童气管插管外,次要安全性结果在每个年龄组中因药物而异。解释 确定性癫痫持续状态的儿童、成人和老年人对左乙拉西坦、磷苯妥英和丙戊酸钠的反应相似,大约一半的患者治疗成功。这三种药物中的任何一种都可以被认为是苯二氮卓类药物难治性癫痫持续状态的潜在首选、二线药物。资助国家神经疾病和中风研究所,美国国立卫生研究院。次要安全性结果在每个年龄组中没有因药物而显着不同。解释 确定性癫痫持续状态的儿童、成人和老年人对左乙拉西坦、磷苯妥英和丙戊酸钠的反应相似,大约一半的患者治疗成功。这三种药物中的任何一种都可以被认为是苯二氮卓类药物难治性癫痫持续状态的潜在首选、二线药物。资助国家神经疾病和中风研究所,美国国立卫生研究院。次要安全性结果在每个年龄组中没有因药物而显着不同。解释 确定性癫痫持续状态的儿童、成人和老年人对左乙拉西坦、磷苯妥英和丙戊酸钠的反应相似,大约一半的患者治疗成功。这三种药物中的任何一种都可以被认为是苯二氮卓类药物难治性癫痫持续状态的潜在首选、二线药物。资助国家神经疾病和中风研究所,美国国立卫生研究院。这三种药物中的任何一种都可以被认为是苯二氮卓类药物难治性癫痫持续状态的潜在首选、二线药物。资助国家神经疾病和中风研究所,美国国立卫生研究院。这三种药物中的任何一种都可以被认为是苯二氮卓类药物难治性癫痫持续状态的潜在首选、二线药物。资助国家神经疾病和中风研究所,美国国立卫生研究院。
更新日期:2020-04-10
中文翻译:

左乙拉西坦、磷苯妥英和丙戊酸钠对按年龄组确定的癫痫持续状态 (ESETT) 的疗效:一项双盲、反应适应性随机对照试验。
背景苯二氮卓类药物难治性或确定的癫痫持续状态被认为在儿童和成人中具有相似的病理生理学,但潜在病因学和药效学的差异可能会不同地影响对治疗的反应。在确定的癫痫持续状态治疗试验 (ESETT) 中,我们比较了左乙拉西坦、磷苯妥英和丙戊酸盐在确定的癫痫持续状态中的疗效和安全性,在这里我们描述了我们在扩大儿童入组后的结果,以比较三个年龄组的结果。方法 在这项多中心、双盲、响应适应性随机对照试验中,我们从全美 58 家医院急诊科招募了患者。如果患者年龄在 2 岁或以上,则他们符合入选条件,已接受足量苯二氮卓类持续时间超过 5 分钟的全身性惊厥发作治疗,并且在最后一次苯二氮卓类药物给药后至少 5 分钟且不超过 30 分钟内在急诊室继续出现持续或反复发作的惊厥。使用贝叶斯方法并按年龄组(<18 岁、18-65 岁和>65 岁)分层,患者以反应适应性方式被随机分配至左乙拉西坦、磷苯妥英或丙戊酸盐。所有患者、研究人员、研究人员和药剂师均对治疗分配设盲。主要结果是在药物输注开始后 1 小时内没有临床明显的癫痫发作,意识得到改善,并且没有额外的抗癫痫药物。主要安全性结果是危及生命的低血压或心律失常。通过意向治疗分析疗效和安全性结果。本研究在 ClinicalTrials.gov 注册,NCT01960075。2015 年 11 月 3 日至 2018 年 12 月 29 日期间,我们招募了 478 名患者,其中包括 462 名独特患者:225 名儿童(年龄 <18 岁)、186 名成人(18-65 岁)和 51 名老年人(>65 岁) ). 175 名 (38%) 患者被随机分配至左乙拉西坦组,142 名 (31%) 至磷苯妥英组,145 名 (31%) 至丙戊酸盐组。基线特征在不同年龄组内的治疗之间是平衡的。在接受左乙拉西坦治疗的儿童中,52%(95% 可信区间 41-62)、44%(33-55)的成人和 37%(19-59)的老年人达到了主要疗效结果;49% (38-61) 的儿童、46% (34-59) 的成人和 35% (17-59) 的老年人使用磷苯妥英;52% (41-63) 的儿童、46% (34-58) 的成年人和 47% (25-70) 的老年人使用丙戊酸盐。在每个年龄组中,药物的疗效或主要安全性结果没有发现差异。除儿童气管插管外,次要安全性结果在每个年龄组中因药物而异。解释 确定性癫痫持续状态的儿童、成人和老年人对左乙拉西坦、磷苯妥英和丙戊酸钠的反应相似,大约一半的患者治疗成功。这三种药物中的任何一种都可以被认为是苯二氮卓类药物难治性癫痫持续状态的潜在首选、二线药物。资助国家神经疾病和中风研究所,美国国立卫生研究院。在每个年龄组中,药物的疗效或主要安全性结果没有发现差异。除儿童气管插管外,次要安全性结果在每个年龄组中因药物而异。解释 确定性癫痫持续状态的儿童、成人和老年人对左乙拉西坦、磷苯妥英和丙戊酸钠的反应相似,大约一半的患者治疗成功。这三种药物中的任何一种都可以被认为是苯二氮卓类药物难治性癫痫持续状态的潜在首选、二线药物。资助国家神经疾病和中风研究所,美国国立卫生研究院。在每个年龄组中,药物的疗效或主要安全性结果没有发现差异。除儿童气管插管外,次要安全性结果在每个年龄组中因药物而异。解释 确定性癫痫持续状态的儿童、成人和老年人对左乙拉西坦、磷苯妥英和丙戊酸钠的反应相似,大约一半的患者治疗成功。这三种药物中的任何一种都可以被认为是苯二氮卓类药物难治性癫痫持续状态的潜在首选、二线药物。资助国家神经疾病和中风研究所,美国国立卫生研究院。次要安全性结果在每个年龄组中没有因药物而显着不同。解释 确定性癫痫持续状态的儿童、成人和老年人对左乙拉西坦、磷苯妥英和丙戊酸钠的反应相似,大约一半的患者治疗成功。这三种药物中的任何一种都可以被认为是苯二氮卓类药物难治性癫痫持续状态的潜在首选、二线药物。资助国家神经疾病和中风研究所,美国国立卫生研究院。次要安全性结果在每个年龄组中没有因药物而显着不同。解释 确定性癫痫持续状态的儿童、成人和老年人对左乙拉西坦、磷苯妥英和丙戊酸钠的反应相似,大约一半的患者治疗成功。这三种药物中的任何一种都可以被认为是苯二氮卓类药物难治性癫痫持续状态的潜在首选、二线药物。资助国家神经疾病和中风研究所,美国国立卫生研究院。这三种药物中的任何一种都可以被认为是苯二氮卓类药物难治性癫痫持续状态的潜在首选、二线药物。资助国家神经疾病和中风研究所,美国国立卫生研究院。这三种药物中的任何一种都可以被认为是苯二氮卓类药物难治性癫痫持续状态的潜在首选、二线药物。资助国家神经疾病和中风研究所,美国国立卫生研究院。


























 京公网安备 11010802027423号
京公网安备 11010802027423号