当前位置:
X-MOL 学术
›
Sol. Energy Mater. Sol. Cells
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Development and performance investigation of MgSO4/SrCl2 composite salt hydrate for mid-low temperature thermochemical heat storage
Solar Energy Materials and Solar Cells ( IF 6.9 ) Pub Date : 2020-06-01 , DOI: 10.1016/j.solmat.2020.110509 Wei Li , Min Zeng , Qiuwang Wang
Solar Energy Materials and Solar Cells ( IF 6.9 ) Pub Date : 2020-06-01 , DOI: 10.1016/j.solmat.2020.110509 Wei Li , Min Zeng , Qiuwang Wang
|
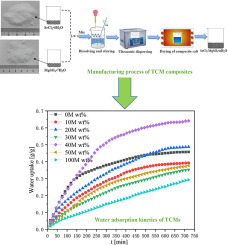
Abstract Salt hydrate based thermochemical heat storage (TCHS) is currently a pivotal technique used for long-term energy storage due to its reversible gas-solid reaction at middle-to-low temperature. MgSO4 is a promising salt candidate owing to its theoretically ultrahigh energy storage density (ESD). However, its poor heat and mass transfer performance severely restricts its large-scale application. Composite salts consist of MgSO4 and other promising salt are prepared to improve the thermochemical performance such as the adsorption kinetics. Simultaneous thermal analysis (STA) is used to screen several other potential salt candidates by comparing the ESD. SrCl2 shows the highest ESD and is selected to mix with MgSO4. X-ray diffraction confirms that the composite salt hydrate is successfully prepared, and the chemical composition is measured by X-ray fluorescence spectrometry. The STA results show that the mixture with an appropriate composition proportion can enhance ESD at mid-low temperatures. The mixture with 20 wt% MgSO4 possesses the best adsorption kinetics even under the most unfavorable condition. Vapor adsorption isobars for dehydrated SrCl2, MgSO4, and their mixtures are measured between 30 °C and 90 °C, which is conducive to understand the chemisorption mechanism. Additionally, cyclability test results show that the aforementioned mixture has better cycle stability than do pure salts. Specifically, the heat storage capacity of the mixture stays above 75% of the original after 20 consecutive heat charging (dehydration) and discharging (hydration) cycles. All these results indicate that the composite salt has good potential for use in long-term TCHS at mid-low temperatures.
中文翻译:

中低温热化学蓄热MgSO4/SrCl2复合盐水合物的研制及性能研究
摘要 盐水合物热化学蓄热(TCHS)在中低温下具有可逆的气固反应,是目前用于长期储能的关键技术。MgSO4 是一种很有前途的盐类候选物,因为它具有理论上超高的能量存储密度 (ESD)。然而,其较差的传热传质性能严重制约了其大规模应用。复合盐由 MgSO4 和其他有前途的盐组成,以改善热化学性能,如吸附动力学。同时热分析 (STA) 用于通过比较 ESD 来筛选其他几种潜在的候选盐。SrCl2 显示出最高的 ESD,并被选择与 MgSO4 混合。X射线衍射证实复合盐水合物制备成功,化学成分通过X射线荧光光谱法测定。STA结果表明,适当组成比例的混合物可以增强中低温下的ESD。即使在最不利的条件下,含有 20 wt% MgSO4 的混合物也具有最佳的吸附动力学。在 30 °C 和 90 °C 之间测量脱水 SrCl2、MgSO4 及其混合物的蒸汽吸附等压线,这有利于了解化学吸附机理。此外,循环性测试结果表明,上述混合物比纯盐具有更好的循环稳定性。具体来说,经过20次连续的充热(脱水)和放热(水化)循环后,混合物的蓄热能力保持在原来的75%以上。
更新日期:2020-06-01
中文翻译:

中低温热化学蓄热MgSO4/SrCl2复合盐水合物的研制及性能研究
摘要 盐水合物热化学蓄热(TCHS)在中低温下具有可逆的气固反应,是目前用于长期储能的关键技术。MgSO4 是一种很有前途的盐类候选物,因为它具有理论上超高的能量存储密度 (ESD)。然而,其较差的传热传质性能严重制约了其大规模应用。复合盐由 MgSO4 和其他有前途的盐组成,以改善热化学性能,如吸附动力学。同时热分析 (STA) 用于通过比较 ESD 来筛选其他几种潜在的候选盐。SrCl2 显示出最高的 ESD,并被选择与 MgSO4 混合。X射线衍射证实复合盐水合物制备成功,化学成分通过X射线荧光光谱法测定。STA结果表明,适当组成比例的混合物可以增强中低温下的ESD。即使在最不利的条件下,含有 20 wt% MgSO4 的混合物也具有最佳的吸附动力学。在 30 °C 和 90 °C 之间测量脱水 SrCl2、MgSO4 及其混合物的蒸汽吸附等压线,这有利于了解化学吸附机理。此外,循环性测试结果表明,上述混合物比纯盐具有更好的循环稳定性。具体来说,经过20次连续的充热(脱水)和放热(水化)循环后,混合物的蓄热能力保持在原来的75%以上。


























 京公网安备 11010802027423号
京公网安备 11010802027423号