当前位置:
X-MOL 学术
›
J. Hepatol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Paneth cells promote angiogenesis and regulate portal hypertension in response to microbial signals
Journal of Hepatology ( IF 25.7 ) Pub Date : 2020-09-01 , DOI: 10.1016/j.jhep.2020.03.019 Mohsin Hassan 1 , Sheida Moghadamrad 2 , Marcel Sorribas 3 , Sergi G Muntet 1 , Philipp Kellmann 1 , Coralie Trentesaux 4 , Marie Fraudeau 4 , Paolo Nanni 5 , Witold Wolski 5 , Irene Keller 6 , Siegfried Hapfelmeier 7 , Noah F Shroyer 8 , Reiner Wiest 9 , Beatrice Romagnolo 4 , Andrea De Gottardi 10
Journal of Hepatology ( IF 25.7 ) Pub Date : 2020-09-01 , DOI: 10.1016/j.jhep.2020.03.019 Mohsin Hassan 1 , Sheida Moghadamrad 2 , Marcel Sorribas 3 , Sergi G Muntet 1 , Philipp Kellmann 1 , Coralie Trentesaux 4 , Marie Fraudeau 4 , Paolo Nanni 5 , Witold Wolski 5 , Irene Keller 6 , Siegfried Hapfelmeier 7 , Noah F Shroyer 8 , Reiner Wiest 9 , Beatrice Romagnolo 4 , Andrea De Gottardi 10
Affiliation
|
BACKGROUND AND AIMS
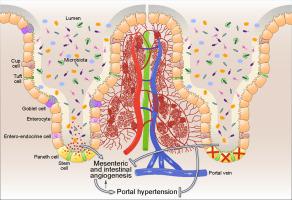
Paneth cells (PCs) synthesize and secrete antimicrobial peptides that are key mediators of host-microbe interactions establishing a balance between intestinal microflora and enteric pathogens. We observed that their number increases in experimental portal hypertension and aimed to investigate the mechanisms by which these cells can contribute to the regulation of portal pressure. METHODS
We first treated Math1Lox/LoxVillcreERT2mice with tamoxifen to induce the complete depletion of intestinal PCs. Subsequently, we performed partial portal vein or bile duct ligation. We then studied the effects of these interventions on hemodynamic parameters, proliferation of blood vessels and the expression of genes regulating angiogenesis. Intestinal organoids were cultured and exposed to different microbial-derived products to study the composition of their secreted products by proteomics and their effects on the proliferation and tube formation of endothelial cells (ECs). In vivo confocal laser endomicroscopy was used to confirm the findings on blood vessel proliferation. RESULTS
Portal hypertension was significantly attenuated in PC-depleted mice compared to control mice and was associated with a decrease in portosystemic shunts. Depletion of PCs also resulted in a significantly decreased density of blood vessels in the intestinal wall and mesentery. Furthermore, we assessed the expression of intestinal genes regulating angiogenesis in Paneth cell depleted mice using arrays and next generation sequencing. Tube formation and wound healing responses were significantly decreased in ECs treated with conditioned media from PC-depleted intestinal organoids exposed to intestinal microbial-derived products. Proteomic analysis of conditioned media in the presence of PCs revealed an increase in factors regulating angiogenesis and additional metabolic processes. In vivo endomicroscopy showed decreased vascular proliferation in absence of PCs. CONCLUSIONS
The in vivo and in vitro results presented here suggest that intestinal flora and microbial-derived factors positively regulate PCs to secrete not only antimicrobial peptides, but also pro-angiogenic signalling molecules, thereby promoting intestinal and mesenteric angiogenesis and regulating portal hypertension.
中文翻译:

潘氏细胞响应微生物信号促进血管生成并调节门静脉高压
背景和目的 Paneth 细胞 (PC) 合成和分泌抗菌肽,这些抗菌肽是宿主-微生物相互作用的关键介质,可在肠道菌群和肠道病原体之间建立平衡。我们观察到它们在实验性门脉高压中的数量增加,旨在研究这些细胞有助于调节门脉压力的机制。方法我们首先用他莫昔芬处理 Math1Lox/LoxVillcreERT2 小鼠以诱导肠道 PC 的完全消耗。随后,我们进行了部分门静脉或胆管结扎术。然后我们研究了这些干预措施对血液动力学参数、血管增殖和调节血管生成基因表达的影响。肠道类器官被培养并暴露于不同的微生物衍生产物,以通过蛋白质组学研究其分泌产物的组成及其对内皮细胞 (ECs) 增殖和管形成的影响。体内共聚焦激光内窥镜用于确认血管增殖的发现。结果 与对照小鼠相比,PC 耗竭小鼠的门脉高压显着减弱,并且与门体分流减少有关。PC 的消耗也导致肠壁和肠系膜血管密度显着降低。此外,我们使用阵列和下一代测序评估了调节 Paneth 细胞耗竭小鼠血管生成的肠道基因的表达。在用来自暴露于肠道微生物衍生产品的 PC 耗尽的肠道类器官的条件培养基处理的 ECs 中,管形成和伤口愈合反应显着降低。在 PC 存在下对条件培养基的蛋白质组学分析显示,调节血管生成和其他代谢过程的因素增加。体内内镜检查显示在没有 PC 的情况下血管增殖减少。结论 这里呈现的体内和体外结果表明,肠道菌群和微生物源性因子正调节 PCs,不仅分泌抗菌肽,还分泌促血管生成信号分子,从而促进肠道和肠系膜血管生成并调节门静脉高压。
更新日期:2020-09-01
中文翻译:

潘氏细胞响应微生物信号促进血管生成并调节门静脉高压
背景和目的 Paneth 细胞 (PC) 合成和分泌抗菌肽,这些抗菌肽是宿主-微生物相互作用的关键介质,可在肠道菌群和肠道病原体之间建立平衡。我们观察到它们在实验性门脉高压中的数量增加,旨在研究这些细胞有助于调节门脉压力的机制。方法我们首先用他莫昔芬处理 Math1Lox/LoxVillcreERT2 小鼠以诱导肠道 PC 的完全消耗。随后,我们进行了部分门静脉或胆管结扎术。然后我们研究了这些干预措施对血液动力学参数、血管增殖和调节血管生成基因表达的影响。肠道类器官被培养并暴露于不同的微生物衍生产物,以通过蛋白质组学研究其分泌产物的组成及其对内皮细胞 (ECs) 增殖和管形成的影响。体内共聚焦激光内窥镜用于确认血管增殖的发现。结果 与对照小鼠相比,PC 耗竭小鼠的门脉高压显着减弱,并且与门体分流减少有关。PC 的消耗也导致肠壁和肠系膜血管密度显着降低。此外,我们使用阵列和下一代测序评估了调节 Paneth 细胞耗竭小鼠血管生成的肠道基因的表达。在用来自暴露于肠道微生物衍生产品的 PC 耗尽的肠道类器官的条件培养基处理的 ECs 中,管形成和伤口愈合反应显着降低。在 PC 存在下对条件培养基的蛋白质组学分析显示,调节血管生成和其他代谢过程的因素增加。体内内镜检查显示在没有 PC 的情况下血管增殖减少。结论 这里呈现的体内和体外结果表明,肠道菌群和微生物源性因子正调节 PCs,不仅分泌抗菌肽,还分泌促血管生成信号分子,从而促进肠道和肠系膜血管生成并调节门静脉高压。



























 京公网安备 11010802027423号
京公网安备 11010802027423号