当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Second Dissociation Constant of Carbonic Acid in H2O and D2O from 150 to 325 °C at p = 21 MPa Using Raman Spectroscopy and a Sapphire-Windowed Flow Cell
The Journal of Physical Chemistry B ( IF 3.3 ) Pub Date : 2020-03-20 , DOI: 10.1021/acs.jpcb.9b11358 Jacy Conrad 1 , Swaroop Sasidharanpillai 1 , Peter R. Tremaine 1
The Journal of Physical Chemistry B ( IF 3.3 ) Pub Date : 2020-03-20 , DOI: 10.1021/acs.jpcb.9b11358 Jacy Conrad 1 , Swaroop Sasidharanpillai 1 , Peter R. Tremaine 1
Affiliation
|
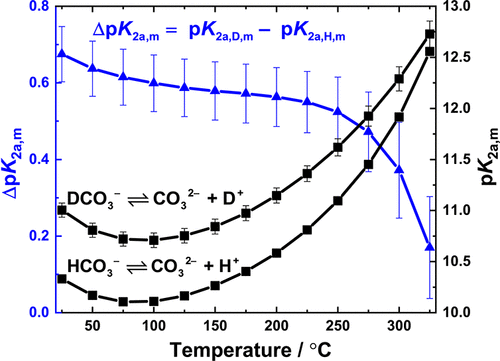
Solvent-corrected reduced isotropic spectra of carbonate and bicarbonate in light and heavy water have been measured from 150 to 325 °C at 21 MPa using a confocal Raman microscope and a custom-built titanium flow cell with sapphire windows. The positions of the symmetric vibrational modes of CO32– and HCO3–/DCO3– were compared to density functional theory (DFT) calculations with a polarizable continuum model in light and heavy water. The experimental Raman peak positions shifted linearly toward lower wavenumbers with increasing temperatures. Raman scattering coefficients, measured relative to a perchlorate internal standard, were used to determine equilibrium molalities of the carbonate and bicarbonate species. These yielded quantitative thermodynamic equilibrium quotients for the reaction CO32– + H2O ⇌ HCO3– + OH– and its deuterium counterpart. Ionization constants for HCO3– and DCO3–, K2a,H,m and K2a,D,m, calculated in their standard states using the Meissner–Tester activity coefficient model, were combined with critically evaluated literature data to derive expressions for their dependence on temperature and pressure, expressed as solvent molar volume, over the range 25 to 325 °C from psat to 21 MPa. These are the first experimental values to be reported for this reaction in light water above 250 °C and in heavy water above 25 °C. The value of the deuterium isotope effect on the chemical equilibrium constant, ΔpK2a,m = pK2a,D,m – pK2a,H,m, decreased from ΔpK2a,m = 0.67 ± 0.07 at 25 °C to ΔpK2a,m = 0.17 ± 0.13 at 325 °C and psat.
中文翻译:

使用拉曼光谱和蓝宝石窗口流通池,在p = 21 MPa时,H 2 O和D 2 O中的碳酸在150至325°C下的第二解离常数
使用共聚焦拉曼显微镜和定制的带蓝宝石窗口的钛流动池,在21 MPa下从150至325°C测量了轻和重水中碳酸盐和碳酸氢盐的溶剂校正还原各向同性光谱。CO 3 2–和HCO 3 – / DCO 3 –的对称振动模式的位置将其与可极化连续体模型在轻水和重水中的密度泛函理论(DFT)计算进行了比较。随着温度的升高,实验拉曼峰的位置向较低波数线性移动。相对于高氯酸盐内标测量的拉曼散射系数用于确定碳酸盐和碳酸氢盐物质的平衡摩尔浓度。这些为反应CO 3 2– + H 2 O⇌HCO 3 – + OH –及其氘代反应提供了定量的热力学平衡商。HCO 3 –和DCO 3 –的电离常数,K 2a,H,m和使用Meissner–Tester活性系数模型以标准状态计算的K 2a,D,m与严格评估的文献数据相结合,得出其对温度和压力的依赖性表达式,表示为溶剂摩尔体积,范围为25至p饱和至21 MPa时为325°C 。这些是在250°C以上的轻水和25°C以上的重水中进行该反应的第一个实验值。对化学平衡常数,ΔP的氘同位素效应值ķ 2A,米= P ķ 2A,d,米- P ķ 2A,H,M,从ΔP降低ķ 2a中,米= 0.67±0.07在25℃下到ΔP ķ在325°C和p sat下,2a,m = 0.17±0.13 。
更新日期:2020-03-21
中文翻译:

使用拉曼光谱和蓝宝石窗口流通池,在p = 21 MPa时,H 2 O和D 2 O中的碳酸在150至325°C下的第二解离常数
使用共聚焦拉曼显微镜和定制的带蓝宝石窗口的钛流动池,在21 MPa下从150至325°C测量了轻和重水中碳酸盐和碳酸氢盐的溶剂校正还原各向同性光谱。CO 3 2–和HCO 3 – / DCO 3 –的对称振动模式的位置将其与可极化连续体模型在轻水和重水中的密度泛函理论(DFT)计算进行了比较。随着温度的升高,实验拉曼峰的位置向较低波数线性移动。相对于高氯酸盐内标测量的拉曼散射系数用于确定碳酸盐和碳酸氢盐物质的平衡摩尔浓度。这些为反应CO 3 2– + H 2 O⇌HCO 3 – + OH –及其氘代反应提供了定量的热力学平衡商。HCO 3 –和DCO 3 –的电离常数,K 2a,H,m和使用Meissner–Tester活性系数模型以标准状态计算的K 2a,D,m与严格评估的文献数据相结合,得出其对温度和压力的依赖性表达式,表示为溶剂摩尔体积,范围为25至p饱和至21 MPa时为325°C 。这些是在250°C以上的轻水和25°C以上的重水中进行该反应的第一个实验值。对化学平衡常数,ΔP的氘同位素效应值ķ 2A,米= P ķ 2A,d,米- P ķ 2A,H,M,从ΔP降低ķ 2a中,米= 0.67±0.07在25℃下到ΔP ķ在325°C和p sat下,2a,m = 0.17±0.13 。


























 京公网安备 11010802027423号
京公网安备 11010802027423号