当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
On The Nature of C(sp3)-C(sp2) Bond Formation in Nickel-Catalyzed Tertiary Radical Cross-Couplings: A Case Study of Ni/Photoredox Catalytic Cross-Coupling of Alkyl Radicals and Aryl Halides
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2020-03-20 , DOI: 10.1021/jacs.0c02355 Mingbin Yuan 1 , Zhihui Song 1 , Shorouk O. Badir 2 , Gary A. Molander 2 , Osvaldo Gutierrez 1
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2020-03-20 , DOI: 10.1021/jacs.0c02355 Mingbin Yuan 1 , Zhihui Song 1 , Shorouk O. Badir 2 , Gary A. Molander 2 , Osvaldo Gutierrez 1
Affiliation
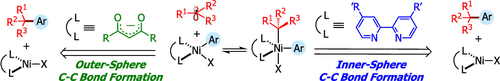
|
The merger of photoredox and nickel catalysis has enabled the construction of quaternary centers. However, the mechanism, role of the ligand, and effect of the spin state for this transformation, and related Ni-catalyzed cross-couplings involving tertiary alkyl radicals in combination with bipyridine and diketonate ligands, remains unknown. Several mechanisms have been proposed, all invoking a key Ni(III) species prior to undergoing irreversible inner-sphere reductive elimination. In this work, we have used open-shell dispersion-corrected DFT calculations, quasi-classical dynamics calculations, and experiments to study in detail the mechanism of carbon-carbon bond formation in Ni bipyridine- and diketonate-based catalytic systems. These calculations revealed that access to high spin states is critical for effective radical cross-coupling of tertiary alkyl radicals. Further, these calculations revealed a disparate mechanism for the C-C bond formation. Specifically, contrary to neutral Ni-bipyridyl system, diketonate ligands lead directly to the corresponding tertiary radical cross-coupling products via an outer-sphere reductive elimination step via triplet spin state from the Ni(III) intermediates. Implications to related Ni-catalyzed radical cross-couplings and the design of new transformations is discussed.
中文翻译:

关于镍催化的叔自由基交叉偶联中 C(sp3)-C(sp2) 键形成的性质:烷基自由基和芳基卤化物的 Ni/Photoredox 催化交叉偶联的案例研究
光氧化还原和镍催化的结合使得四元中心的构建成为可能。然而,这种转变的机制、配体的作用和自旋态的影响,以及涉及叔烷基与联吡啶和二酮配体组合的相关 Ni 催化交叉偶联,仍然未知。已经提出了几种机制,所有机制都在进行不可逆的内球还原消除之前调用关键的 Ni(III) 物种。在这项工作中,我们使用开壳色散校正 DFT 计算、准经典动力学计算和实验来详细研究 Ni 联吡啶和二酮基催化体系中碳 - 碳键形成的机制。这些计算表明,获得高自旋态对于叔烷基自由基的有效自由基交叉偶联至关重要。此外,这些计算揭示了 CC 键形成的不同机制。具体而言,与中性 Ni-联吡啶体系相反,二酮配体通过来自 Ni(III) 中间体的三重自旋态通过外球还原消除步骤直接导致相应的叔自由基交叉偶联产物。讨论了对相关镍催化自由基交叉偶联和新转化设计的影响。二酮配体通过来自 Ni(III) 中间体的三重自旋态通过外球还原消除步骤直接导致相应的叔自由基交叉偶联产物。讨论了对相关镍催化自由基交叉偶联和新转化设计的影响。二酮配体通过来自 Ni(III) 中间体的三重自旋态通过外球还原消除步骤直接导致相应的叔自由基交叉偶联产物。讨论了对相关镍催化自由基交叉偶联和新转化设计的影响。
更新日期:2020-03-20
中文翻译:

关于镍催化的叔自由基交叉偶联中 C(sp3)-C(sp2) 键形成的性质:烷基自由基和芳基卤化物的 Ni/Photoredox 催化交叉偶联的案例研究
光氧化还原和镍催化的结合使得四元中心的构建成为可能。然而,这种转变的机制、配体的作用和自旋态的影响,以及涉及叔烷基与联吡啶和二酮配体组合的相关 Ni 催化交叉偶联,仍然未知。已经提出了几种机制,所有机制都在进行不可逆的内球还原消除之前调用关键的 Ni(III) 物种。在这项工作中,我们使用开壳色散校正 DFT 计算、准经典动力学计算和实验来详细研究 Ni 联吡啶和二酮基催化体系中碳 - 碳键形成的机制。这些计算表明,获得高自旋态对于叔烷基自由基的有效自由基交叉偶联至关重要。此外,这些计算揭示了 CC 键形成的不同机制。具体而言,与中性 Ni-联吡啶体系相反,二酮配体通过来自 Ni(III) 中间体的三重自旋态通过外球还原消除步骤直接导致相应的叔自由基交叉偶联产物。讨论了对相关镍催化自由基交叉偶联和新转化设计的影响。二酮配体通过来自 Ni(III) 中间体的三重自旋态通过外球还原消除步骤直接导致相应的叔自由基交叉偶联产物。讨论了对相关镍催化自由基交叉偶联和新转化设计的影响。二酮配体通过来自 Ni(III) 中间体的三重自旋态通过外球还原消除步骤直接导致相应的叔自由基交叉偶联产物。讨论了对相关镍催化自由基交叉偶联和新转化设计的影响。


























 京公网安备 11010802027423号
京公网安备 11010802027423号