当前位置:
X-MOL 学术
›
Eur. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Studies towards the total synthesis of drimentine C. Preparation of the AB and CDEF ring fragments
European Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2020-04-17 , DOI: 10.1002/ejoc.202000158 Sarah M. Pound 1 , Steven J. Underwood 1 , Christopher J. Douglas 1
European Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2020-04-17 , DOI: 10.1002/ejoc.202000158 Sarah M. Pound 1 , Steven J. Underwood 1 , Christopher J. Douglas 1
Affiliation
|
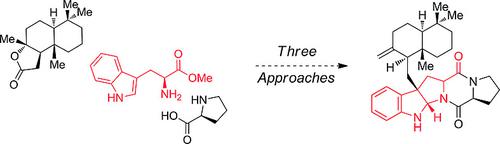
The drimentine family is a class of hybrid isoprenoids derived from actinomycete bacteria. Members of this family display weak antitumor and antibacterial activity. Herein we report our efforts toward the total synthesis of drimentine C using three distinct approaches incorporating palladium-catalyzed cyanoamidation, reductive cross-coupling, and photoredox-catalyzed α-alkylation of an aldehyde as key steps. Our synthetic efforts use a convergent synthesis to assemble the terpenoid and alkaloid portions of drimentine C from readily available l-tryptophan, l-proline, and (+)-sclareolide.
中文翻译:

对drimentine C的全合成研究。AB和CDEF环片段的制备
drimentine 家族是一类源自放线菌的杂合类异戊二烯。该家族的成员显示出较弱的抗肿瘤和抗菌活性。在此,我们报告了我们使用三种不同的方法全合成 drimentine C 的努力,这些方法包括钯催化的氰基酰胺化、还原交叉偶联和光氧化还原催化的醛的 α-烷基化作为关键步骤。我们的合成工作使用收敛合成法从现成的 l-色氨酸、l-脯氨酸和 (+)-sclareolide 中组装 drimentine C 的萜类和生物碱部分。
更新日期:2020-04-17
中文翻译:

对drimentine C的全合成研究。AB和CDEF环片段的制备
drimentine 家族是一类源自放线菌的杂合类异戊二烯。该家族的成员显示出较弱的抗肿瘤和抗菌活性。在此,我们报告了我们使用三种不同的方法全合成 drimentine C 的努力,这些方法包括钯催化的氰基酰胺化、还原交叉偶联和光氧化还原催化的醛的 α-烷基化作为关键步骤。我们的合成工作使用收敛合成法从现成的 l-色氨酸、l-脯氨酸和 (+)-sclareolide 中组装 drimentine C 的萜类和生物碱部分。



























 京公网安备 11010802027423号
京公网安备 11010802027423号