当前位置:
X-MOL 学术
›
J. Hazard. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Ethanol introduced synthesis of ultrastable 1T-MoS2 for removal of Cr(VI).
Journal of Hazardous Materials ( IF 13.6 ) Pub Date : 2020-03-19 , DOI: 10.1016/j.jhazmat.2020.122525 Zeyang Li 1 , Ruoyu Fan 1 , Zhi Hu 1 , Wenchao Li 1 , Hongjian Zhou 2 , Shenghong Kang 2 , Yunxia Zhang 2 , Haimin Zhang 2 , Guozhong Wang 1
Journal of Hazardous Materials ( IF 13.6 ) Pub Date : 2020-03-19 , DOI: 10.1016/j.jhazmat.2020.122525 Zeyang Li 1 , Ruoyu Fan 1 , Zhi Hu 1 , Wenchao Li 1 , Hongjian Zhou 2 , Shenghong Kang 2 , Yunxia Zhang 2 , Haimin Zhang 2 , Guozhong Wang 1
Affiliation
|
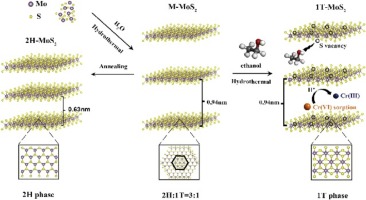
Metallic 1T phase of MoS2 (1T-MoS2) has aroused great concern for decontamination of heavy metal ions from water. Herein, ultrastable 1T-MoS2 was successfully achieved via a gentle two-stage solvothermal strategy utilizing water and ethanol as solvent for efficient removal of Cr(VI). Notably, nearly 100 % 1T-MoS2 was obtained, and it remained highly stable in air even for 360 days. Electron paramagnetic resonance analysis showed that sulfur vacancies were in situ formed on the 1T/2H mixed phase MoS2 (M-MoS2) under the induction of ethanol, which is critical to promote the transformation of 2H to 1T phase. Molecular dynamic simulation revealed that there was strong interaction between ethanol and MoS2 surface, which could decrease the total energy of MoS2 for strengthening stability of 1T phase. Moreover, 1T-MoS2 shows superior sorption capacity (200.3 mg·g-1) for removal of Cr(VI), twice more than that of M-MoS2 and 2H phase MoS2 under the same condition. Significantly, the stable phase structure of 1T-MoS2 and chromium adsorption capacity still remained even after five cycles of chromium adsorption. The study of Cr(VI) adsorption mechanism revealed that the chromium adsorption was attributed to the undercoordinated Mo(IV) as active site and coupled with redox reaction during removal process.
中文翻译:

乙醇引入了用于去除Cr(VI)的超稳定1T-MoS2的合成。
MoS2的金属1T相(1T-MoS2)引起了对水中重金属离子净化的极大关注。在此,通过使用水和乙醇作为溶剂有效去除Cr(VI)的温和两步溶剂热策略成功实现了超稳定的1T-MoS2。值得注意的是,获得了接近100%的1T-MoS2,即使在360天的空气中也保持高度稳定。电子顺磁共振分析表明,在乙醇的诱导下,1T / 2H混合相MoS2(M-MoS2)上原位形成了硫空位,这对于促进2H向1T相的转化至关重要。分子动力学模拟表明,乙醇与MoS2表面存在强相互作用,可以降低MoS2的总能量,从而增强1T相的稳定性。此外,在相同条件下,1T-MoS2对Cr(VI)的去除具有优异的吸附能力(200.3 mg·g-1),是M-MoS2和2H相MoS2的两倍。值得注意的是,即使经过五次铬吸附循环,1T-MoS2的稳定相结构和铬吸附能力仍然保持。对Cr(VI)吸附机理的研究表明,铬的吸附归因于Mo(IV)配位不足作为活性位点,并且在去除过程中与氧化还原反应有关。
更新日期:2020-03-20
中文翻译:

乙醇引入了用于去除Cr(VI)的超稳定1T-MoS2的合成。
MoS2的金属1T相(1T-MoS2)引起了对水中重金属离子净化的极大关注。在此,通过使用水和乙醇作为溶剂有效去除Cr(VI)的温和两步溶剂热策略成功实现了超稳定的1T-MoS2。值得注意的是,获得了接近100%的1T-MoS2,即使在360天的空气中也保持高度稳定。电子顺磁共振分析表明,在乙醇的诱导下,1T / 2H混合相MoS2(M-MoS2)上原位形成了硫空位,这对于促进2H向1T相的转化至关重要。分子动力学模拟表明,乙醇与MoS2表面存在强相互作用,可以降低MoS2的总能量,从而增强1T相的稳定性。此外,在相同条件下,1T-MoS2对Cr(VI)的去除具有优异的吸附能力(200.3 mg·g-1),是M-MoS2和2H相MoS2的两倍。值得注意的是,即使经过五次铬吸附循环,1T-MoS2的稳定相结构和铬吸附能力仍然保持。对Cr(VI)吸附机理的研究表明,铬的吸附归因于Mo(IV)配位不足作为活性位点,并且在去除过程中与氧化还原反应有关。

























 京公网安备 11010802027423号
京公网安备 11010802027423号