当前位置:
X-MOL 学术
›
J. Chromatogr. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Quantification of residual hydrophobic fusion peptide with monomer and dimer forms using reversed-phase liquid chromatography.
Journal of Chromatography B ( IF 3 ) Pub Date : 2020-03-19 , DOI: 10.1016/j.jchromb.2020.122073 Cindy X Cai 1 , Nicole A Schneck 1 , Vera B Ivleva 1 , Krishana Gulla 1 , Yaqiu Zhang 1 , Daniel Gowetski 1 , Q Paula Lei 1
Journal of Chromatography B ( IF 3 ) Pub Date : 2020-03-19 , DOI: 10.1016/j.jchromb.2020.122073 Cindy X Cai 1 , Nicole A Schneck 1 , Vera B Ivleva 1 , Krishana Gulla 1 , Yaqiu Zhang 1 , Daniel Gowetski 1 , Q Paula Lei 1
Affiliation
|
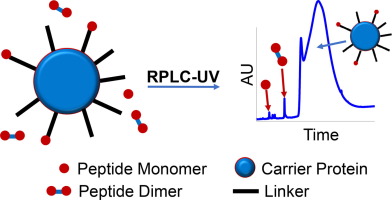
A fusion peptide mimicking a part of the sequence of HIV-1 envelope glycoprotein with an additional cysteine at its C-terminus (FP8: AVGIGAVFC) was conjugated to a carrier protein through a linker for development of an HIV-1 vaccine. Since this fusion peptide is very hydrophobic with poor solubility and can self-dimerize via a disulfide bond, co-existence of monomeric and dimeric forms presented a major challenge for residual unconjugated FP8 quantification. A reversed-phase liquid chromatography (RPLC) with UV detection was developed to monitor residual FP8 using an experimental correction factor of 0.85 for UV peak area measurement between FP8 dimer and monomer. Therefore, both forms of unconjugated residual FP8 can be measured based on a single FP8 monomer reference curve. Overall, this study demonstrated that the current purification process can remove free residual FP8 to a low level, <20 µg/mL, which showed negligible impact (<10%) for the conjugated FP8 ratio measurement using another method, amino acid analysis.
中文翻译:

使用反相液相色谱定量残留的具有单体和二聚体形式的疏水融合肽。
通过连接子将模拟HIV-1包膜糖蛋白序列的一部分的融合肽在其C末端带有一个额外的半胱氨酸(FP8:AVGIGAVFC)与载体蛋白偶联,以开发HIV-1疫苗。由于该融合肽疏水性很强,溶解性较差,并且可以通过二硫键自二聚,因此单体和二聚体形式的共存是残留未缀合FP8定量分析的主要挑战。开发了一种具有紫外检测功能的反相液相色谱(RPLC),以使用实验校正因子0.85监测FP8二聚体和单体之间的UV峰面积,从而监测残留的FP8。因此,可以基于单个FP8单体参考曲线测量两种形式的未结合残留FP8。总体,
更新日期:2020-03-20
中文翻译:

使用反相液相色谱定量残留的具有单体和二聚体形式的疏水融合肽。
通过连接子将模拟HIV-1包膜糖蛋白序列的一部分的融合肽在其C末端带有一个额外的半胱氨酸(FP8:AVGIGAVFC)与载体蛋白偶联,以开发HIV-1疫苗。由于该融合肽疏水性很强,溶解性较差,并且可以通过二硫键自二聚,因此单体和二聚体形式的共存是残留未缀合FP8定量分析的主要挑战。开发了一种具有紫外检测功能的反相液相色谱(RPLC),以使用实验校正因子0.85监测FP8二聚体和单体之间的UV峰面积,从而监测残留的FP8。因此,可以基于单个FP8单体参考曲线测量两种形式的未结合残留FP8。总体,


























 京公网安备 11010802027423号
京公网安备 11010802027423号