Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy ( IF 4.4 ) Pub Date : 2020-03-19 , DOI: 10.1016/j.saa.2020.118279 N. Anastassova , S. Stoyanov , A. Mavrova , D. Yancheva
|
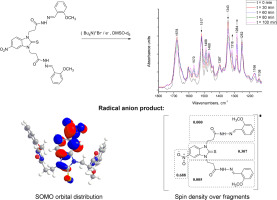
The conversion of N,N′-disubstituted hydrazone derivatives of 5-nitrobenzimidazole-2-thione into radical anion and dianion products was studied through infrared (IR) spectroscopy and computational methods. The electrochemical reduction of 3,3′-(5-nitro-2-thioxo-1H-benzo[d]imidazole-1,3(2H)-diyl)bis(N′-(2-methoxybenzylidene))propane-hydrazide was performed directly in the IR cell and the spectral changes were monitored over time in order to identify the spectral bands originating from the reduction product. In order to clarify whether the reduction leads to the generation of radical anion or deprotonated radical dianion, a second spectroscopic experiment was carried out where deprotonation was achieved by treatment with sodium methoxide. Both experiments resulted in distinctly different spectral features, giving evidence that the reduction to radical anion is not accompanied by deprotonation. In order to explain the experimentally observed differences in the hepatotoxicity within the series of N,N′-disubstituted derivatives of 5-nitrobenzimidazole-2-thione, several molecular electronic parameters such as frontier molecular orbitals, spin and charge distribution over fragments, and electron affinities of the studied hydrazone derivatives were compared to those of a previously studied ester derivative. Based on the estimated electronic parameters, it was shown that the type of the side chains (ester, hydrazone etc.) attached to the N-atoms in the nitrobenzimidazole derivatives do not change significantly the propensity of the compounds towards nitro reduction, but however the generated radical anions are characterized by different reactivity accounting for the different hepatotoxicity.
中文翻译:

5-硝基苯并咪唑-2-硫酮的N,N'-二取代衍生物转化为阴离子和自由基阴离子产物的光谱和计算机模拟研究:对肝毒性的影响
通过红外光谱和计算方法研究了5-硝基苯并咪唑-2-硫酮的N,N'-二取代衍生物向自由基阴离子和二阴离子产物的转化。3,3'-(5-硝基-2-硫代-1H-苯并[ d]的电化学还原直接在红外池中进行]咪唑-1,3(2H)-二基)双(N'-(2-甲氧基亚苄基))丙烷-酰肼的监测,并随时间监测光谱变化,以鉴定源自以下物质的光谱带还原产品。为了弄清楚还原是导致自由基阴离子的生成还是使去质子化的自由基二价阴离子发生,进行了第二次光谱实验,其中通过用甲醇钠处理实现了去质子化。两次实验均产生明显不同的光谱特征,从而证明自由基阴离子的还原不会伴随去质子化。为了解释实验观察到的一系列5-硝基苯并咪唑-2-硫酮的N,N'-二取代衍生物在肝毒性方面的差异,将几个molecular分子衍生物的分子电子参数,例如前沿分子轨道,自旋和电荷分布以及所研究的were衍生物的电子亲和力与先前研究的酯衍生物进行了比较。根据估算的电子参数,表明与硝基苯并咪唑衍生物的N原子连接的侧链类型(酯,等)不会显着改变化合物硝基还原的倾向,但是产生的自由基阴离子的特征在于不同的反应性,这解释了不同的肝毒性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号