当前位置:
X-MOL 学术
›
Enzyme Microb. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Characterization of a recombinant D-mannose-producing D-lyxose isomerase from Caldanaerobius polysaccharolyticus
Enzyme and Microbial Technology ( IF 3.4 ) Pub Date : 2020-08-01 , DOI: 10.1016/j.enzmictec.2020.109553 Hao Wu 1 , Ming Chen 1 , Cuie Guang 1 , Wenli Zhang 1 , Wanmeng Mu 2
Enzyme and Microbial Technology ( IF 3.4 ) Pub Date : 2020-08-01 , DOI: 10.1016/j.enzmictec.2020.109553 Hao Wu 1 , Ming Chen 1 , Cuie Guang 1 , Wenli Zhang 1 , Wanmeng Mu 2
Affiliation
|
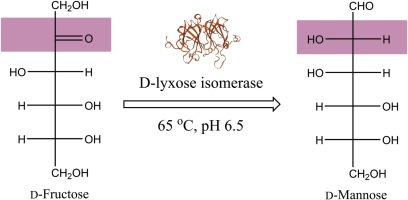
Recently, functional sugars, such as d-mannose, have attracted considerable attention due to their excellent physiological benefits for human health and wide applications in food and pharmaceutical industries. Therefore, d-mannose production using a sugar isomerase such as d-lyxose isomerase (d-LIase) has emerged as a research hotspot owing to its advantages over plant extraction and chemical synthesis methods. In this study, a putative d-LIase gene from Caldanaerobius polysaccharolyticus was cloned and expressed in Escherichia coli. Then, a biochemical characterization of the recombinant d-LIase was carried out and its potential use in d-mannose production also assessed. Results showed that d-LIase exhibited its maximum activity under these optimal conditions: temperature of 65 °C, a pH of 6.5, and the Mn2+ metal ion. The d-LIase was active at pH 6.0-8.0; it was also quite thermostable up to 60 °C and approximately 85 % of its maximum activity was retained after incubating for 4 h. Further, our Nano-DSC analysis determined that its melting temperature (Tm) was 70.74 °C. Using 100, 300, and 500 g L-1 of d-fructose as substrate, 25.6, 74.4, and 115 g L-1 of d-mannose were produced respectively, corresponding to a conversion rate of 25.6 %, 24.8 %, and 23.0 % under optimal conditions. Taken together, our results provide evidence for a promising candidate d-LIase for producing d-mannose directly from d-fructose.
中文翻译:

来自 Caldanaerobius polysaccharolyticus 的重组 D-甘露糖生产 D-来苏糖异构酶的表征
近年来,功能性糖类,如 d-甘露糖,因其对人体健康的优异生理益处以及在食品和制药行业的广泛应用而备受关注。因此,利用糖异构酶如 d-来苏糖异构酶 (d-LIase) 生产 d-甘露糖因其优于植物提取和化学合成方法的优势而成为研究热点。在这项研究中,来自 Caldanaerobius polysaccharolyticus 的假定 d-LIase 基因被克隆并在大肠杆菌中表达。然后,对重组 d-LIase 进行了生化表征,并评估了其在 d-甘露糖生产中的潜在用途。结果表明,d-LIase 在以下最佳条件下表现出最大活性:温度为 65 °C,pH 值为 6.5,Mn2+ 金属离子。d-LIase 在 pH 6.0-8.0 时有活性;它在高达 60 °C 的温度下也非常稳定,并且在孵育 4 小时后仍保留了其最大活性的约 85%。此外,我们的纳米 DSC 分析确定其熔化温度 (Tm) 为 70.74 °C。使用100、300和500 g L-1的d-果糖作为底物,分别产生25.6、74.4和115 g L-1的d-甘露糖,对应的转化率分别为25.6 %、24.8 %和23.0 % 在最佳条件下。总之,我们的结果为直接从 d-果糖生产 d-甘露糖的有希望的候选 d-LIase 提供了证据。以 500 g L-1 d-果糖为底物,分别产生 25.6、74.4 和 115 g L-1 d-甘露糖,对应的最佳条件下转化率为 25.6%、24.8% 和 23.0%。总之,我们的结果为直接从 d-果糖生产 d-甘露糖的有希望的候选 d-LIase 提供了证据。以 500 g L-1 d-果糖为底物,分别产生 25.6、74.4 和 115 g L-1 d-甘露糖,对应的最佳条件下转化率为 25.6%、24.8% 和 23.0%。总之,我们的结果为直接从 d-果糖生产 d-甘露糖的有希望的候选 d-LIase 提供了证据。
更新日期:2020-08-01
中文翻译:

来自 Caldanaerobius polysaccharolyticus 的重组 D-甘露糖生产 D-来苏糖异构酶的表征
近年来,功能性糖类,如 d-甘露糖,因其对人体健康的优异生理益处以及在食品和制药行业的广泛应用而备受关注。因此,利用糖异构酶如 d-来苏糖异构酶 (d-LIase) 生产 d-甘露糖因其优于植物提取和化学合成方法的优势而成为研究热点。在这项研究中,来自 Caldanaerobius polysaccharolyticus 的假定 d-LIase 基因被克隆并在大肠杆菌中表达。然后,对重组 d-LIase 进行了生化表征,并评估了其在 d-甘露糖生产中的潜在用途。结果表明,d-LIase 在以下最佳条件下表现出最大活性:温度为 65 °C,pH 值为 6.5,Mn2+ 金属离子。d-LIase 在 pH 6.0-8.0 时有活性;它在高达 60 °C 的温度下也非常稳定,并且在孵育 4 小时后仍保留了其最大活性的约 85%。此外,我们的纳米 DSC 分析确定其熔化温度 (Tm) 为 70.74 °C。使用100、300和500 g L-1的d-果糖作为底物,分别产生25.6、74.4和115 g L-1的d-甘露糖,对应的转化率分别为25.6 %、24.8 %和23.0 % 在最佳条件下。总之,我们的结果为直接从 d-果糖生产 d-甘露糖的有希望的候选 d-LIase 提供了证据。以 500 g L-1 d-果糖为底物,分别产生 25.6、74.4 和 115 g L-1 d-甘露糖,对应的最佳条件下转化率为 25.6%、24.8% 和 23.0%。总之,我们的结果为直接从 d-果糖生产 d-甘露糖的有希望的候选 d-LIase 提供了证据。以 500 g L-1 d-果糖为底物,分别产生 25.6、74.4 和 115 g L-1 d-甘露糖,对应的最佳条件下转化率为 25.6%、24.8% 和 23.0%。总之,我们的结果为直接从 d-果糖生产 d-甘露糖的有希望的候选 d-LIase 提供了证据。


























 京公网安备 11010802027423号
京公网安备 11010802027423号