Bioorganic & Medicinal Chemistry Letters ( IF 2.7 ) Pub Date : 2020-03-19 , DOI: 10.1016/j.bmcl.2020.127118 Yanguo Shang , Qingjing Hao , Kaixuan Jiang , Mengting He , Jinxin Wang
|
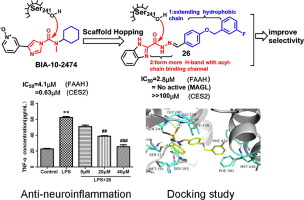
Fatty acid amide hydrolase (FAAH) is a promising target for the development of drugs to treat pain, inflammation, and other central nervous system disorders. Herein, a series of novel heterocyclic carbohydrazide derivatives were firstly designed by the classic scaffold-hopping strategy. Then, multi-steps synthesis and human FAAH enzyme inhibiting activity assays were conducted. Among them, compound 26 showed strong inhibition against human FAAH with IC50 of 2.8 μM. Corresponding docking studies revealed that the acyl hydrazide group of compound 26 well-occupied the acyl-chain binding pocket. It also exhibited high selectivity towards FAAH when comparing with CES2 and MAGL. Additionally, compound 26 effectively suppressed the LPS-induced neuroinflammation of microglial cells (BV2) via the reduction of interleukin-1β and tumor necrosis factor-α. Our results provided significative lead compounds for the further discovery of novel selective and safe FAAH inhibitors with potent anti-neuroinflammation activity.
中文翻译:

杂环碳酰肼衍生物作为新型选择性脂肪酸酰胺水解酶抑制剂的发现:设计,合成和抗神经炎性评估
Fatty acid amide hydrolase (FAAH) is a promising target for the development of drugs to treat pain, inflammation, and other central nervous system disorders. Herein, a series of novel heterocyclic carbohydrazide derivatives were firstly designed by the classic scaffold-hopping strategy. Then, multi-steps synthesis and human FAAH enzyme inhibiting activity assays were conducted. Among them, compound 26 showed strong inhibition against human FAAH with IC50 of 2.8 μM. Corresponding docking studies revealed that the acyl hydrazide group of compound 26 well-occupied the acyl-chain binding pocket. It also exhibited high selectivity towards FAAH when comparing with CES2 and MAGL. Additionally, compound 26通过减少白介素-1β和肿瘤坏死因子-α有效抑制LPS诱导的小胶质细胞(BV2)神经炎。我们的结果为进一步发现具有有效抗神经炎症活性的新型选择性和安全FAAH抑制剂提供了重要的先导化合物。


























 京公网安备 11010802027423号
京公网安备 11010802027423号