当前位置:
X-MOL 学术
›
Tetrahedron Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Gold Catalyzed Cascade Reaction of 2-Alkynyl Arylazides with 1,2,3-Triazoles to Provide N1- and N2-Indol-3-yl 1,2,3-Triazole derivatives
Tetrahedron Letters ( IF 1.8 ) Pub Date : 2020-03-19 , DOI: 10.1016/j.tetlet.2020.151851 Tao Li , Bai-Ling Chen , Li-Li Zhu , Zili Chen
中文翻译:

金催化2-炔基芳基叠氮化物与1,2,3-三唑的级联反应,以提供N 1-和N 2 -Indol-3-yl 1,2,3-三唑衍生物
更新日期:2020-03-20
Tetrahedron Letters ( IF 1.8 ) Pub Date : 2020-03-19 , DOI: 10.1016/j.tetlet.2020.151851 Tao Li , Bai-Ling Chen , Li-Li Zhu , Zili Chen
|
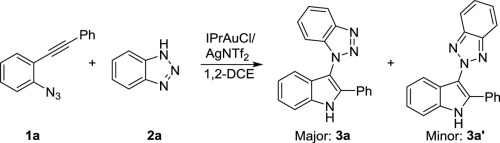
A new method was developed to prepare N1 and N2-indol-3-yl 1,2,3-trizole products through gold catalyzed cascade reaction of o-alkynyl arylazides with 1,2,3-triazoles, in which, the in-situ generated α-imino gold carbene intermediate was intercepted by various types of triazole compounds. N1-selective nucleophilic attack was favored to give moderate to high N1/N2 selectivity. In addition, indol-3-yl pyrazole compounds were also prepared by using the similar method.
中文翻译:

金催化2-炔基芳基叠氮化物与1,2,3-三唑的级联反应,以提供N 1-和N 2 -Indol-3-yl 1,2,3-三唑衍生物
的新方法的开发是为了准备Ñ 1和Ñ 2 -通过金催化级联反应吲哚-3-基-1,2,3-三唑产品ø -炔基芳基叠氮化用的1,2,3-三唑,其中,所述在原位生成的α-亚氨基金卡宾中间体被各种类型的三唑化合物拦截。N 1-选择性亲核攻击有利于产生中等至高的N 1 / N 2选择性。另外,吲哚-3-基吡唑化合物也通过使用类似方法制备。


























 京公网安备 11010802027423号
京公网安备 11010802027423号