当前位置:
X-MOL 学术
›
ACS Infect. Dis.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Substrate Specificity of OXA-48 after β5-β6 Loop Replacement.
ACS Infectious Diseases ( IF 5.3 ) Pub Date : 2020-03-19 , DOI: 10.1021/acsinfecdis.9b00452 Laura Dabos 1, 2 , Agustin Zavala 3 , Rémy A Bonnin 1, 2, 4 , Oliver Beckstein 5 , Pascal Retailleau 3 , Bogdan I Iorga 3 , Thierry Naas 1, 2, 4, 6
ACS Infectious Diseases ( IF 5.3 ) Pub Date : 2020-03-19 , DOI: 10.1021/acsinfecdis.9b00452 Laura Dabos 1, 2 , Agustin Zavala 3 , Rémy A Bonnin 1, 2, 4 , Oliver Beckstein 5 , Pascal Retailleau 3 , Bogdan I Iorga 3 , Thierry Naas 1, 2, 4, 6
Affiliation
|
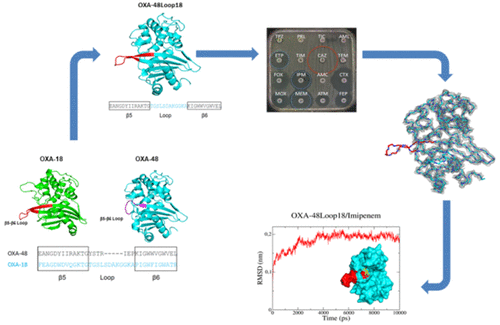
OXA-48 carbapenemase has rapidly spread in many countries worldwide with several OXA-48-variants being described, differing by a few amino acid (AA) substitutions or deletions, mostly in the β5-β6 loop. While single AA substitutions have only a minor impact on OXA-48 hydrolytic profiles, others with 4 AA deletions result in loss of carbapenem hydrolysis and gain of expanded-spectrum cephalosporin (ESC) hydrolysis. We have replaced the β5-β6 loop of OXA-48 with that of OXA-18, a clavulanic-acid inhibited oxacillinase capable of hydrolyzing ESCs but not carbapenems. The hybrid enzyme OXA-48Loop18 was able to hydrolyze ESCs and carbapenems (although with a lower kcat), even though the β5-β6 loop was longer and its sequence quite different from that of OXA-48. The kinetic parameters of OXA-48Loop18 were in agreement with the MIC values. X-ray crystallography and molecular modeling suggest that the conformation of the grafted loop allows the binding of bulkier substrates, unlike that of the native loop, expanding the hydrolytic profile. This seems to be due not only to differences in AA sequence, but also to the backbone conformation the loop can adopt. Finally, our results provide further experimental evidence for the role of the β5-β6 loop in substrate selectivity of OXA-48-like enzymes and additional details on the structure-function relationship of β-lactamases, demonstrating how localized changes in these proteins can alter or expand their function, highlighting their plasticity.
中文翻译:

β5-β6环置换后OXA-48的底物特异性。
OXA-48碳青霉烯酶在全球许多国家迅速传播,描述了几种OXA-48变体,区别在于一些氨基酸(AA)取代或缺失,主要是在β5-β6环中。虽然单个AA取代对OXA-48水解曲线影响很小,但其他具有4个AA缺失的取代会导致碳青霉烯水解作用丧失,并获得广谱头孢菌素(ESC)水解作用。我们用OXA-18取代了OXA-48的β5-β6环,OXA-18是一种棒酸抑制的草酸酶,能够水解ESC,但不能水解碳青霉烯。杂合酶OXA-48Loop18能够水解ESC和碳青霉烯(尽管kcat较低),即使β5-β6环更长且其序列与OXA-48完全不同。OXA-48Loop18的动力学参数与MIC值一致。X射线晶体学和分子模型表明,接枝环的构象允许结合更大的底物,而不是天然环,从而扩展了水解特性。这似乎不仅是由于AA序列的差异,还归因于该环可以采用的主链构象。最后,我们的结果为β5-β6环在OXA-48-样酶的底物选择性中的作用以及β-内酰胺酶的结构-功能关系的其他细节提供了进一步的实验证据,证明了这些蛋白质中的局部变化如何改变或扩展其功能,突出其可塑性。这似乎不仅是由于AA序列的差异,还归因于该环可以采用的主链构象。最后,我们的结果为β5-β6环在OXA-48-样酶的底物选择性中的作用以及β-内酰胺酶的结构-功能关系的其他细节提供了进一步的实验证据,证明了这些蛋白质中的局部变化如何改变或扩展其功能,突出其可塑性。这似乎不仅是由于AA序列的差异,还归因于该环可以采用的主链构象。最后,我们的结果为β5-β6环在OXA-48-样酶的底物选择性中的作用以及β-内酰胺酶的结构-功能关系的其他细节提供了进一步的实验证据,证明了这些蛋白质中的局部变化如何改变或扩展其功能,突出其可塑性。
更新日期:2020-03-10
中文翻译:

β5-β6环置换后OXA-48的底物特异性。
OXA-48碳青霉烯酶在全球许多国家迅速传播,描述了几种OXA-48变体,区别在于一些氨基酸(AA)取代或缺失,主要是在β5-β6环中。虽然单个AA取代对OXA-48水解曲线影响很小,但其他具有4个AA缺失的取代会导致碳青霉烯水解作用丧失,并获得广谱头孢菌素(ESC)水解作用。我们用OXA-18取代了OXA-48的β5-β6环,OXA-18是一种棒酸抑制的草酸酶,能够水解ESC,但不能水解碳青霉烯。杂合酶OXA-48Loop18能够水解ESC和碳青霉烯(尽管kcat较低),即使β5-β6环更长且其序列与OXA-48完全不同。OXA-48Loop18的动力学参数与MIC值一致。X射线晶体学和分子模型表明,接枝环的构象允许结合更大的底物,而不是天然环,从而扩展了水解特性。这似乎不仅是由于AA序列的差异,还归因于该环可以采用的主链构象。最后,我们的结果为β5-β6环在OXA-48-样酶的底物选择性中的作用以及β-内酰胺酶的结构-功能关系的其他细节提供了进一步的实验证据,证明了这些蛋白质中的局部变化如何改变或扩展其功能,突出其可塑性。这似乎不仅是由于AA序列的差异,还归因于该环可以采用的主链构象。最后,我们的结果为β5-β6环在OXA-48-样酶的底物选择性中的作用以及β-内酰胺酶的结构-功能关系的其他细节提供了进一步的实验证据,证明了这些蛋白质中的局部变化如何改变或扩展其功能,突出其可塑性。这似乎不仅是由于AA序列的差异,还归因于该环可以采用的主链构象。最后,我们的结果为β5-β6环在OXA-48-样酶的底物选择性中的作用以及β-内酰胺酶的结构-功能关系的其他细节提供了进一步的实验证据,证明了这些蛋白质中的局部变化如何改变或扩展其功能,突出其可塑性。

























 京公网安备 11010802027423号
京公网安备 11010802027423号