当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Multifunctional Electrocatalysts: Ru–M (M = Co, Ni, Fe) for Alkaline Fuel Cells and Electrolyzers
ACS Catalysis ( IF 12.9 ) Pub Date : 2020-03-19 , DOI: 10.1021/acscatal.9b05621 Hongsen Wang 1 , Yao Yang 1 , Francis J. DiSalvo 1 , Héctor D. Abruña 1
ACS Catalysis ( IF 12.9 ) Pub Date : 2020-03-19 , DOI: 10.1021/acscatal.9b05621 Hongsen Wang 1 , Yao Yang 1 , Francis J. DiSalvo 1 , Héctor D. Abruña 1
Affiliation
|
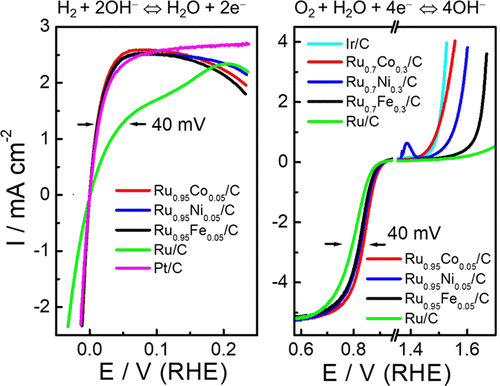
Moving from proton exchange membrane fuel cells to anion exchange membrane fuel cells (AEMFCs) enables the use of non-Pt-group (NPG) metals as cathodes for the oxygen reduction reaction, since the oxygen reduction kinetics on NPG metals is significantly enhanced in alkaline media. These NPG metal catalysts are also stable under alkaline conditions and cost much less than Pt-group metals. However, in alkaline media, H2 oxidation on Pt anodes is much more sluggish than in acidic media, and thus, more active H2 oxidation catalysts are required to enable AEMFCs. Here we report on a family of H2 oxidation catalysts: Ru alloys with Co, Ni, or Fe. A series of RuCo/C, RuNi/C, and RuFe/C alloy nanoparticle catalysts have been synthesized via an impregnation method and characterized by atomic-scale scanning transmission electron microscopy. We find that Ru alloys with small amounts of Co, Ni, or Fe can significantly enhance H2 oxidation (HOR), H2 evolution (HER), O2 reduction (ORR), and oxygen evolution (OER) reactions in alkaline media. They are much more active than pure Ru catalysts for the HOR, HER, ORR, and OER, and even more active than pure Pt catalysts for the HOR and HER, but they cost much less. In particular, Ru0.95Co0.05/C is the most active among all studied Ru alloys catalysts for the HOR, HER, and ORR. Thus, they are promising catalysts for alkaline fuel cells and electrolyzers. The enhancement mechanism of Ru alloys has been elucidated by density functional theory calculations.
中文翻译:

多功能电催化剂:Ru–M(M = Co,Ni,Fe)用于碱性燃料电池和电解槽
从质子交换膜燃料电池转移到阴离子交换膜燃料电池(AEMFC),可以将非Pt(NPG)金属用作氧还原反应的阴极,因为在碱性条件下,NPG金属上的氧还原动力学显着增强媒体。这些NPG金属催化剂在碱性条件下也很稳定,而且成本远低于Pt类金属。但是,在碱性介质中,Pt阳极上的H 2氧化比在酸性介质中要慢得多,因此,需要更多活性H 2氧化催化剂才能制成AEMFC。在这里我们报告一个H 2族氧化催化剂:含Co,Ni或Fe的Ru合金。通过浸渍法合成了一系列RuCo / C,RuNi / C和RuFe / C合金纳米颗粒催化剂,并通过原子尺度扫描透射电子显微镜进行了表征。我们发现,在碱性介质中,具有少量Co,Ni或Fe的Ru合金可以显着增强H 2氧化(HOR),H 2析出(HER),O 2还原(ORR)和氧析出(OER)反应。对于HOR,HER,ORR和OER,它们比纯Ru催化剂更具活性,对于HOR和HER,它们比纯Pt催化剂更具活性,但它们的成本要低得多。特别是Ru 0.95 Co 0.05/ C是所有研究的HOR,HER和ORR钌合金催化剂中活性最高的催化剂。因此,它们是用于碱性燃料电池和电解槽的有前途的催化剂。通过密度泛函理论计算已经阐明了钌合金的增强机理。
更新日期:2020-04-23
中文翻译:

多功能电催化剂:Ru–M(M = Co,Ni,Fe)用于碱性燃料电池和电解槽
从质子交换膜燃料电池转移到阴离子交换膜燃料电池(AEMFC),可以将非Pt(NPG)金属用作氧还原反应的阴极,因为在碱性条件下,NPG金属上的氧还原动力学显着增强媒体。这些NPG金属催化剂在碱性条件下也很稳定,而且成本远低于Pt类金属。但是,在碱性介质中,Pt阳极上的H 2氧化比在酸性介质中要慢得多,因此,需要更多活性H 2氧化催化剂才能制成AEMFC。在这里我们报告一个H 2族氧化催化剂:含Co,Ni或Fe的Ru合金。通过浸渍法合成了一系列RuCo / C,RuNi / C和RuFe / C合金纳米颗粒催化剂,并通过原子尺度扫描透射电子显微镜进行了表征。我们发现,在碱性介质中,具有少量Co,Ni或Fe的Ru合金可以显着增强H 2氧化(HOR),H 2析出(HER),O 2还原(ORR)和氧析出(OER)反应。对于HOR,HER,ORR和OER,它们比纯Ru催化剂更具活性,对于HOR和HER,它们比纯Pt催化剂更具活性,但它们的成本要低得多。特别是Ru 0.95 Co 0.05/ C是所有研究的HOR,HER和ORR钌合金催化剂中活性最高的催化剂。因此,它们是用于碱性燃料电池和电解槽的有前途的催化剂。通过密度泛函理论计算已经阐明了钌合金的增强机理。



























 京公网安备 11010802027423号
京公网安备 11010802027423号