当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
CanE, an Iron/2-Oxoglutarate-Dependent Lasso Peptide Hydroxylase from Streptomyces canus.
ACS Chemical Biology ( IF 4 ) Pub Date : 2020-03-30 , DOI: 10.1021/acschembio.0c00109 Chen Zhang 1 , Mohammad R Seyedsayamdost 1, 2
ACS Chemical Biology ( IF 4 ) Pub Date : 2020-03-30 , DOI: 10.1021/acschembio.0c00109 Chen Zhang 1 , Mohammad R Seyedsayamdost 1, 2
Affiliation
|
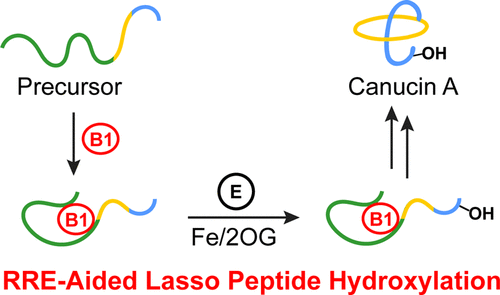
Lasso peptides are a class of ribosomally synthesized and post-translationally modified peptides (RiPPs) that feature a unique lariat-knot topology. Canucin A, a post-translationally hydroxylated lasso peptide, was recently discovered via activation of its otherwise silent biosynthetic gene cluster in Streptomyces canus. The biosynthesis of canucin A, notably the introduction of a hydroxyl group at the β-carbon of the terminal aspartate residue, is the topic of the current report. We combine genetic and biochemical experiments to show that an iron/2-oxoglutarate-dependent enzyme, CanE, installs the hydroxyl group onto the precursor peptide in vivo and in vitro. Moreover, we show that hydroxylation occurs prior to macrocyclization and that the RiPP recognition element (RRE), encoded within the gene cluster to facilitate the initial proteolytic reaction, also increases the yield of hydroxylation, hinting at a dual role for the RRE. Our results have implications for the combinatorial biosynthesis of lasso peptides.
中文翻译:

CanE,来自链霉菌的铁/ 2-氧戊二酸酯依赖性套索肽羟化酶。
套索肽是一类具有独特套索结拓扑结构的核糖体合成和翻译后修饰的肽(RiPP)。Canucin A,一种翻译后羟基化的套索肽,最近通过激活链霉菌中原本沉默的生物合成基因簇而被发现。canucin A的生物合成,特别是在末端天冬氨酸残基的β-碳上引入羟基是本报告的主题。我们结合了遗传和生化实验,表明在体内和体外,铁/ 2-氧戊二酸依赖性酶CanE将羟基安装到前体肽上。此外,我们表明羟基化发生在大环化之前,并且RiPP识别元件(RRE)在基因簇内被编码以促进最初的蛋白水解反应,还增加了羟基化的产率,暗示了RRE的双重作用。我们的结果对套索肽的组合生物合成有影响。
更新日期:2020-04-23
中文翻译:

CanE,来自链霉菌的铁/ 2-氧戊二酸酯依赖性套索肽羟化酶。
套索肽是一类具有独特套索结拓扑结构的核糖体合成和翻译后修饰的肽(RiPP)。Canucin A,一种翻译后羟基化的套索肽,最近通过激活链霉菌中原本沉默的生物合成基因簇而被发现。canucin A的生物合成,特别是在末端天冬氨酸残基的β-碳上引入羟基是本报告的主题。我们结合了遗传和生化实验,表明在体内和体外,铁/ 2-氧戊二酸依赖性酶CanE将羟基安装到前体肽上。此外,我们表明羟基化发生在大环化之前,并且RiPP识别元件(RRE)在基因簇内被编码以促进最初的蛋白水解反应,还增加了羟基化的产率,暗示了RRE的双重作用。我们的结果对套索肽的组合生物合成有影响。



























 京公网安备 11010802027423号
京公网安备 11010802027423号