当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
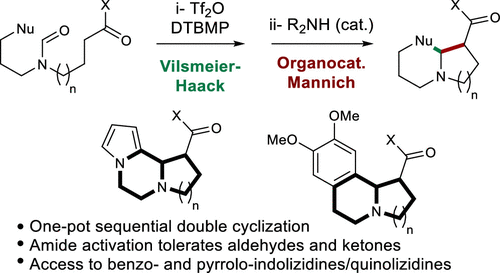
Sequential One-Pot Vilsmeier–Haack and Organocatalyzed Mannich Cyclizations to Functionalized Benzoindolizidines and Benzoquinolizidines
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2020-03-19 , DOI: 10.1021/acs.joc.9b03449 Johanne Outin 1 , Pauline Quellier 1 , Guillaume Bélanger 1
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2020-03-19 , DOI: 10.1021/acs.joc.9b03449 Johanne Outin 1 , Pauline Quellier 1 , Guillaume Bélanger 1
Affiliation
|
The development of new one-pot sequential cyclizations involving a Vilsmeier–Haack reaction followed by an organocatalyzed Mannich reaction is reported. This synthetic strategy gives access to functionalized indolizidines and quinolizidines in one operation from readily synthesized precursors. Yields and diastereoselectivities are good to excellent when formamides are used to trigger the key step, bearing either an electron-rich aryl or a pyrrole as the nucleophilic partner in the first cyclization.
中文翻译:

顺序一锅法Vilsmeier-Haack和有机催化的曼尼希环化作用到功能化的苯并吲哚并核苷和苯并喹啉并核苷
据报道,新的一锅顺序环化反应涉及Vilsmeier-Haack反应,然后进行有机催化的Mannich反应。这种合成策略可通过易于合成的前体在一次操作中获得官能化的吲哚嗪和喹啉嗪。当使用甲酰胺触发关键步骤时,产率和非对映选择性很好,甚至极好,在第一个环化反应中带有富电子芳基或吡咯作为亲核伴侣。
更新日期:2020-03-20
中文翻译:

顺序一锅法Vilsmeier-Haack和有机催化的曼尼希环化作用到功能化的苯并吲哚并核苷和苯并喹啉并核苷
据报道,新的一锅顺序环化反应涉及Vilsmeier-Haack反应,然后进行有机催化的Mannich反应。这种合成策略可通过易于合成的前体在一次操作中获得官能化的吲哚嗪和喹啉嗪。当使用甲酰胺触发关键步骤时,产率和非对映选择性很好,甚至极好,在第一个环化反应中带有富电子芳基或吡咯作为亲核伴侣。

























 京公网安备 11010802027423号
京公网安备 11010802027423号