当前位置:
X-MOL 学术
›
Arch. Pharm.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
New thiazolyl-pyrazoline derivatives bearing nitrogen mustard as potential antimicrobial and antiprotozoal agents
Archiv der Pharmazie ( IF 5.1 ) Pub Date : 2020-03-18 , DOI: 10.1002/ardp.201900351 Viviana Cuartas 1, 2 , Sara M Robledo 3 , Iván D Vélez 3 , María Del Pilar Crespo 4 , Maximiliano Sortino 5 , Susana Zacchino 5 , Manuel Nogueras 6 , Justo Cobo 6 , Yulieth Upegui 3 , Tatiana Pineda 3 , Lina Yepes 3 , Braulio Insuasty 1, 2
Archiv der Pharmazie ( IF 5.1 ) Pub Date : 2020-03-18 , DOI: 10.1002/ardp.201900351 Viviana Cuartas 1, 2 , Sara M Robledo 3 , Iván D Vélez 3 , María Del Pilar Crespo 4 , Maximiliano Sortino 5 , Susana Zacchino 5 , Manuel Nogueras 6 , Justo Cobo 6 , Yulieth Upegui 3 , Tatiana Pineda 3 , Lina Yepes 3 , Braulio Insuasty 1, 2
Affiliation
|
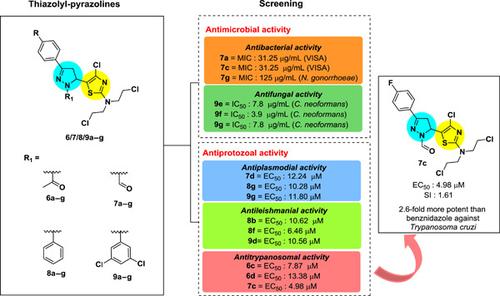
A new series of N‐substituted pyrazoline derivatives 6a–g, 7a–g, 8a–g, and 9a–g was synthetized by reaction of hydrazine derivatives and chalcone–thiazole hybrids bearing nitrogen mustard 5a–g. The chalcones 5a–g were obtained by Claisen–Schmidt condensation of thiazole‐2‐nitrogen mustard 3 and selected acetophenones 4a–g. These new compounds 6/7/8/9a–g were screened for their antifungal activity against Cryptococcus neoformans, with IC50 values of 3.9–7.8 µg/ml for the N‐3,5‐dichlorophenyl pyrazolines 9e–g. Interestingly, those compounds show low cytotoxic effects toward erythrocytes (RBC). In addition, N‐acetyl (6a,b) and N‐formyl pyrazolines (7a, 7b, 7c, and 7g) showed inhibitory activity against methicillin‐susceptible Staphylococcus aureus, methicillin‐resistant S. aureus, and vancomycin‐intermediate S. aureus, with the most important minimum inhibitory concentration values ranging from 31.25 to 125 µg/ml. Regarding the antiprotozoal activity, thiazolyl‐pyrazolines 9g, 8f, and 7c display high activity against Plasmodium falciparum, Leishmania (V) panamensis, and Trypanosoma cruzi, with EC50 values of 11.80, 6.46, and 4.98 μM, respectively, and with 7c being approximately 2.6‐fold more potent than benznidazole with a selectivity index of 1.61 on U‐937 human cells, showing promising potential as a novel antitrypanosomal agent.
中文翻译:

含氮芥的新型噻唑基-吡唑啉衍生物作为潜在的抗菌剂和抗原生动物剂
通过肼衍生物和带有氮芥 5a-g 的查耳酮-噻唑杂化物反应合成了一系列新的 N 取代吡唑啉衍生物 6a-g、7a-g、8a-g 和 9a-g。查耳酮 5a-g 是通过噻唑-2-氮芥 3 和选定的苯乙酮 4a-g 的 Claisen-Schmidt 缩合获得的。筛选了这些新化合物 6/7/8/9a-g 对新型隐球菌的抗真菌活性,N-3,5-二氯苯基吡唑啉 9e-g 的 IC50 值为 3.9-7.8 µg/ml。有趣的是,这些化合物对红细胞 (RBC) 显示出低细胞毒性作用。此外,N-乙酰基(6a、b)和 N-甲酰基吡唑啉(7a、7b、7c 和 7g)对甲氧西林敏感的金黄色葡萄球菌、耐甲氧西林的金黄色葡萄球菌和万古霉素中间体的金黄色葡萄球菌具有抑制活性, 最重要的最低抑菌浓度值为 31.25 至 125 µg/ml。关于抗原生动物活性,噻唑基-吡唑啉 9g、8f 和 7c 对恶性疟原虫、利什曼原虫 (V) 和克氏锥虫显示出高活性,EC50 值分别为 11.80、6.46 和 4.98 μM,大约为比苯并硝唑强 2.6 倍,对 U-937 人体细胞的选择性指数为 1.61,显示出作为新型抗锥虫病药物的潜力。
更新日期:2020-03-18
中文翻译:

含氮芥的新型噻唑基-吡唑啉衍生物作为潜在的抗菌剂和抗原生动物剂
通过肼衍生物和带有氮芥 5a-g 的查耳酮-噻唑杂化物反应合成了一系列新的 N 取代吡唑啉衍生物 6a-g、7a-g、8a-g 和 9a-g。查耳酮 5a-g 是通过噻唑-2-氮芥 3 和选定的苯乙酮 4a-g 的 Claisen-Schmidt 缩合获得的。筛选了这些新化合物 6/7/8/9a-g 对新型隐球菌的抗真菌活性,N-3,5-二氯苯基吡唑啉 9e-g 的 IC50 值为 3.9-7.8 µg/ml。有趣的是,这些化合物对红细胞 (RBC) 显示出低细胞毒性作用。此外,N-乙酰基(6a、b)和 N-甲酰基吡唑啉(7a、7b、7c 和 7g)对甲氧西林敏感的金黄色葡萄球菌、耐甲氧西林的金黄色葡萄球菌和万古霉素中间体的金黄色葡萄球菌具有抑制活性, 最重要的最低抑菌浓度值为 31.25 至 125 µg/ml。关于抗原生动物活性,噻唑基-吡唑啉 9g、8f 和 7c 对恶性疟原虫、利什曼原虫 (V) 和克氏锥虫显示出高活性,EC50 值分别为 11.80、6.46 和 4.98 μM,大约为比苯并硝唑强 2.6 倍,对 U-937 人体细胞的选择性指数为 1.61,显示出作为新型抗锥虫病药物的潜力。

























 京公网安备 11010802027423号
京公网安备 11010802027423号