当前位置:
X-MOL 学术
›
ChemPhysChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
S-H…O and O-H…O Hydrogen Bonds-Comparison of Dimers of Thiocarboxylic and Carboxylic Acids.
ChemPhysChem ( IF 2.9 ) Pub Date : 2020-07-08 , DOI: 10.1002/cphc.202000131 Mohammad Aarabi 1 , Samira Gholami 2 , Sławomir J Grabowski 3, 4
ChemPhysChem ( IF 2.9 ) Pub Date : 2020-07-08 , DOI: 10.1002/cphc.202000131 Mohammad Aarabi 1 , Samira Gholami 2 , Sławomir J Grabowski 3, 4
Affiliation
|
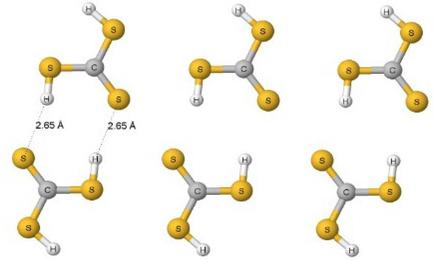
ωB97‐XD/aug‐cc‐pVTZ calculations were performed on dimers of selected thiocarboxylic acids and on analogous carboxylic acids. The sample of calculated thiocarboxylic acids is an extension of the Cambridge Structural Database search that contains only a few such structures. The Natural Bond Orbital (NBO) method, Symmetry‐Adapted Perturbation Theory (SAPT) approach, Non‐Covalent Interaction (NCI) method and Quantum Theory of Atoms in Molecules (QTAIM) were applied additionally to analyse interactions in dimers of thiocarboxylic and carboxylic acids. The insights into crystal structures as well as into results of calculations show that the formation of S−H…O hydrogen bonds between molecules of thiocarboxylic acids is steered by the same mechanisms as the formation of much stronger O−H…O hydrogen bonds in carboxylic acids. The intramolecular O−H…O and C−H…S hydrogen bonds occurring in few considered structures are also analysed.
中文翻译:

SH…O和OH…O氢键-硫代羧酸和羧酸二聚体的比较。
ωB97-XD/ aug-cc-pVTZ计算是针对所选硫代羧酸和类似羧酸的二聚体进行的。计算出的硫代羧酸样品是剑桥结构数据库搜索的扩展,该搜索仅包含一些此类结构。此外,还使用了自然键轨道(NBO)方法,对称自适应扰动理论(SAPT)方法,非共价相互作用(NCI)方法和分子中原子的量子理论(QTAIM)来分析硫代羧酸和羧酸二聚体中的相互作用。 。对晶体结构以及计算结果的洞察表明,硫代羧酸分子之间S-H…O氢键的形成与形成羧基中更强的O-H…O氢键的机理相同。酸。
更新日期:2020-07-08
中文翻译:

SH…O和OH…O氢键-硫代羧酸和羧酸二聚体的比较。
ωB97-XD/ aug-cc-pVTZ计算是针对所选硫代羧酸和类似羧酸的二聚体进行的。计算出的硫代羧酸样品是剑桥结构数据库搜索的扩展,该搜索仅包含一些此类结构。此外,还使用了自然键轨道(NBO)方法,对称自适应扰动理论(SAPT)方法,非共价相互作用(NCI)方法和分子中原子的量子理论(QTAIM)来分析硫代羧酸和羧酸二聚体中的相互作用。 。对晶体结构以及计算结果的洞察表明,硫代羧酸分子之间S-H…O氢键的形成与形成羧基中更强的O-H…O氢键的机理相同。酸。



























 京公网安备 11010802027423号
京公网安备 11010802027423号