当前位置:
X-MOL 学术
›
ChemPhysChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Anion-Anion Complexes Established between Aspartate Dimers.
ChemPhysChem ( IF 2.9 ) Pub Date : 2020-04-14 , DOI: 10.1002/cphc.201901200 Matias O Miranda 1, 2 , Darío J R Duarte 1, 2 , Ibon Alkorta 3
ChemPhysChem ( IF 2.9 ) Pub Date : 2020-04-14 , DOI: 10.1002/cphc.201901200 Matias O Miranda 1, 2 , Darío J R Duarte 1, 2 , Ibon Alkorta 3
Affiliation
|
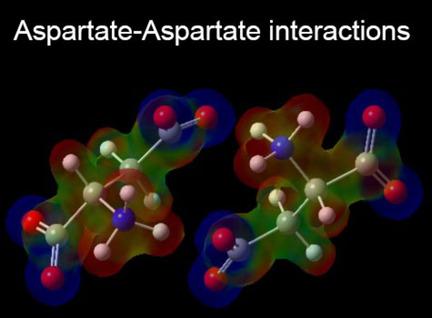
Stable dimers aspartate‐aspartate have been studied in aqueous and gas phase through theoretical simulations. The polarizable continuum model (PCM) has been applied to simulate the effect of the hydration on monomers and complexes. The quantum theory of atoms in molecules (QTAIM) and the interacting quantum atoms (IQA) scheme has been used to inquire into if, in the aqueous phase, individual hydrogen bonds have attractive electrostatic components. In all cases a spontaneous formation of the complexes in the aqueous phase are observed, while in the gas phase a considerable energy barrier must be overcome (between 100.8 to 263.2 kJ mol−1). The intermolecular distance at which this barrier is indicates when the hydrogen‐bond interactions begin to take importance between the dimers and the corresponding molecular recognition among them. The IQA analysis shows that in aqueous phase, the hydrogen bonds N−H⋅⋅⋅O are mainly electrostatic in nature with a certain covalent character which increases linearly with the decrease of internuclear distances H⋅⋅⋅O. The H⋅⋅⋅H interactions observed are stabilizing and they are mainly quantum in nature.
中文翻译:

天冬氨酸二聚体之间建立的阴离子-阴离子络合物。
稳定的二聚体天冬氨酸-天冬氨酸已通过理论模拟在水相和气相中进行了研究。可极化连续体模型(PCM)已用于模拟水合对单体和配合物的影响。分子中原子的量子理论(QTAIM)和相互作用的量子原子(IQA)方案已用于研究在水相中单个氢键是否具有吸引人的静电成分。在所有情况下,观察到在水相中自发形成络合物,而在气相中必须克服相当大的能垒(介于100.8至263.2 kJ mol -1之间)。当二键之间的氢键相互作用开始变得重要时,在该分子之间的分子间距离就很重要。IQA分析表明,在水相中,氢键N-H···O主要是静电的,具有一定的共价特性,随着核距H···O的减小而线性增加。观察到的H⋅⋅⋅H相互作用稳定,并且本质上主要是量子相互作用。
更新日期:2020-04-14
中文翻译:

天冬氨酸二聚体之间建立的阴离子-阴离子络合物。
稳定的二聚体天冬氨酸-天冬氨酸已通过理论模拟在水相和气相中进行了研究。可极化连续体模型(PCM)已用于模拟水合对单体和配合物的影响。分子中原子的量子理论(QTAIM)和相互作用的量子原子(IQA)方案已用于研究在水相中单个氢键是否具有吸引人的静电成分。在所有情况下,观察到在水相中自发形成络合物,而在气相中必须克服相当大的能垒(介于100.8至263.2 kJ mol -1之间)。当二键之间的氢键相互作用开始变得重要时,在该分子之间的分子间距离就很重要。IQA分析表明,在水相中,氢键N-H···O主要是静电的,具有一定的共价特性,随着核距H···O的减小而线性增加。观察到的H⋅⋅⋅H相互作用稳定,并且本质上主要是量子相互作用。


























 京公网安备 11010802027423号
京公网安备 11010802027423号