当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Insights into N-heterocyclic carbene and Lewis acid cooperatively catalyzed oxidative [3 + 3] annulation reactions of α,β-unsaturated aldehyde with 1,3-dicarbonyl compounds
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2020-03-19 , DOI: 10.1039/d0qo00091d Xinghua Wang 1, 2, 3, 4, 5 , Yang Wang 6, 7, 8, 9 , Jinshuai Song 1, 2, 3, 4, 5 , Donghui Wei 1, 2, 3, 4, 5
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2020-03-19 , DOI: 10.1039/d0qo00091d Xinghua Wang 1, 2, 3, 4, 5 , Yang Wang 6, 7, 8, 9 , Jinshuai Song 1, 2, 3, 4, 5 , Donghui Wei 1, 2, 3, 4, 5
Affiliation
|
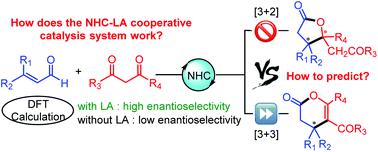
Unravelling the origin of stereoselectivity and chemoselectivity is one of the most challenging questions in the field of N-heterocyclic carbene (NHC) catalysis. Recently, Lewis acid (LA) additives were found to be key additives for improving the stereoselectivity in many NHC-catalyzed annulation reactions (especially [3 + 3] annulation) of dicarbonyl compounds; however, the reason for this general phenomenon remains unclear so far. In order to solve this issue, a universal model has been suggested for NHC and Lewis acid cooperatively catalyzed oxidative [3 + 3] annulation reactions between α,β-unsaturated aldehyde and 1,3-dicarbonyl compounds to explore the origin of the improved stereoselectivity. The computed results indicate that the Lewis acid can increase the free energy difference (ΔΔG‡) between the transition states involved in the stereoselectivity-determining step to improve stereoselectivity, which is mainly due to the stronger non-covalent interactions between the LA-coordinated substrate and the chiral NHC catalyst in the favorable R-isomer transition state. Furthermore, two new reactivity indices, i.e. electrophilic and nucleophilic atom energies (denoted Ea+ and Ea−), were proposed to measure the electrophilicity and nucleophilicity of possible reactive sites, which could be used to predict the chemoselective products in the [3 + 3] and [3 + 2] annulations. The obtained insights will be helpful for the rational design of organocatalytic reactions with excellent stereoselectivity and special chemoselectivity.
中文翻译:

对N-杂环卡宾和路易斯酸协同催化α,β-不饱和醛与1,3-二羰基化合物的氧化[3 + 3]环化反应的见解
揭示立体选择性和化学选择性的起源是N-杂环卡宾(NHC)催化领域最具挑战性的问题之一。最近,发现路易斯酸(LA)添加剂是改善许多NHC催化的二羰基化合物的环合反应(尤其是[3 + 3]环合)中立体选择性的关键添加剂;但是,到目前为止,这种普遍现象的原因尚不清楚。为了解决这个问题,已经提出了NHC和路易斯酸在α,β-不饱和醛与1,3-二羰基化合物之间协同催化的氧化[3 + 3]环氧化反应的通用模型,以探索改进的立体选择性的起源。 。所计算的结果表明,该路易斯酸可以增加自由能差(ΔΔ ģ ‡在确定立体选择性的步骤中涉及的过渡态之间进行改进以提高立体选择性,这主要是由于在R异构体过渡态良好的情况下,LA配位的底物与手性NHC催化剂之间的非共价相互作用更强。此外,两个新的反应性指数,即电和亲核原子的能量(表示为Ë一个+和ë一个-),建议用于测量可能的反应位点的亲电子性和亲核性,可用于预测[3 + 3]和[3 + 2]环空中的化学选择性产物。获得的见解将有助于合理设计具有出色的立体选择性和特殊的化学选择性的有机催化反应。
更新日期:2020-03-19
中文翻译:

对N-杂环卡宾和路易斯酸协同催化α,β-不饱和醛与1,3-二羰基化合物的氧化[3 + 3]环化反应的见解
揭示立体选择性和化学选择性的起源是N-杂环卡宾(NHC)催化领域最具挑战性的问题之一。最近,发现路易斯酸(LA)添加剂是改善许多NHC催化的二羰基化合物的环合反应(尤其是[3 + 3]环合)中立体选择性的关键添加剂;但是,到目前为止,这种普遍现象的原因尚不清楚。为了解决这个问题,已经提出了NHC和路易斯酸在α,β-不饱和醛与1,3-二羰基化合物之间协同催化的氧化[3 + 3]环氧化反应的通用模型,以探索改进的立体选择性的起源。 。所计算的结果表明,该路易斯酸可以增加自由能差(ΔΔ ģ ‡在确定立体选择性的步骤中涉及的过渡态之间进行改进以提高立体选择性,这主要是由于在R异构体过渡态良好的情况下,LA配位的底物与手性NHC催化剂之间的非共价相互作用更强。此外,两个新的反应性指数,即电和亲核原子的能量(表示为Ë一个+和ë一个-),建议用于测量可能的反应位点的亲电子性和亲核性,可用于预测[3 + 3]和[3 + 2]环空中的化学选择性产物。获得的见解将有助于合理设计具有出色的立体选择性和特殊的化学选择性的有机催化反应。


























 京公网安备 11010802027423号
京公网安备 11010802027423号