当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Photochemical α-carboxyalkylation of tryptophols and tryptamines via C-H functionalization.
Chemical Communications ( IF 4.9 ) Pub Date : 2020-04-02 , DOI: 10.1039/d0cc00847h Zhiqiang Pan 1 , Yuchang Liu , Fengchi Hu , Qinglong Liu , Wenbin Shang , Chengfeng Xia
Chemical Communications ( IF 4.9 ) Pub Date : 2020-04-02 , DOI: 10.1039/d0cc00847h Zhiqiang Pan 1 , Yuchang Liu , Fengchi Hu , Qinglong Liu , Wenbin Shang , Chengfeng Xia
Affiliation
|
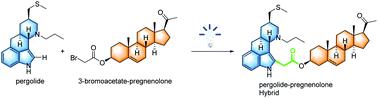
A process for the α-carboxyalkylation of tryptophols and tryptamines by the functionalization of C-H bonds under visible light irradiation has been developed. The photochemical strategy activated the C-Br bonds of α-bromo-alkylcarboxylic esters to provide carbon-centered radicals under the catalysis of Ir(iii) photocatalyst and coupled with indole derivatives. This methodology displayed wide functional group tolerance and excellent regioselectivity, and was applied to the late-stage functionalization and preparation of indole-containing hybrids.
中文翻译:

色胺醇和色胺通过CH官能化的光化学α-羧烷基化。
已经开发了在可见光照射下通过CH键的官能化使色酚和色胺的α-羧烷基化的方法。该光化学策略在Ir(iii)光催化剂的催化下并与吲哚衍生物偶联,活化了α-溴代烷基羧酸酯的C-Br键以提供以碳为中心的自由基。该方法显示出宽泛的官能团耐受性和优异的区域选择性,并应用于后期功能化和含吲哚杂种的制备。
更新日期:2020-03-19
中文翻译:

色胺醇和色胺通过CH官能化的光化学α-羧烷基化。
已经开发了在可见光照射下通过CH键的官能化使色酚和色胺的α-羧烷基化的方法。该光化学策略在Ir(iii)光催化剂的催化下并与吲哚衍生物偶联,活化了α-溴代烷基羧酸酯的C-Br键以提供以碳为中心的自由基。该方法显示出宽泛的官能团耐受性和优异的区域选择性,并应用于后期功能化和含吲哚杂种的制备。


























 京公网安备 11010802027423号
京公网安备 11010802027423号