American Journal of Kidney Diseases ( IF 13.2 ) Pub Date : 2020-03-19 , DOI: 10.1053/j.ajkd.2019.11.012 Pajaree Krisanapan 1 , Surachet Vongsanim 1 , Pathomporn Pin-On 2 , Chidchanok Ruengorn 3 , Kajohnsak Noppakun 4
|
Rationale & Objective
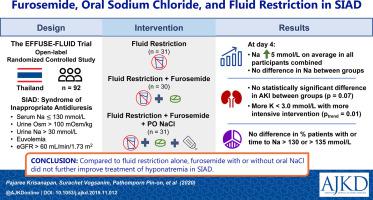
First-line therapy for syndrome of inappropriate antidiuresis (SIAD) is fluid restriction. Additional treatment for patients who do not respond to fluid restriction are water restriction with furosemide or water restriction with furosemide and salt supplementation. However, the efficacy of these treatments has never been tested in a randomized controlled study. The objective of this study was to investigate whether, combined with fluid restriction, furosemide with or without sodium chloride (NaCl) supplementation was more effective than fluid restriction alone in the treatment of hyponatremia in SIAD.
Study Design
Open-label randomized controlled study.
Setting & Participants
Patients with serum sodium concentrations ([Na+]) ≤ 130 mmol/L due to SIAD.
Intervention(s)
Random assignment to 1 of 3 groups: fluid restriction alone (FR), fluid restriction and furosemide (FR+FM), or fluid restriction, furosemide, and NaCl (FR+FM+NaCl). Strictness of fluid restriction (<1,000 or <500 mL/d) was guided by the urine to serum electrolyte ratio. Furosemide dosage was 20 to 40 mg/d. NaCl supplements were 3 g/d. All treatments were continued for 28 days.
Outcomes
The primary outcome was change in [Na+] at days 4, 7, 14, and 28 after randomization.
Results
92 patients were recruited (FR, n = 31; FR+FM, n = 30; FR+FM+NaCl, n = 31). Baseline [Na+] was 125 ± 4 mmol/L, and there were no significant differences between groups. Mean [Na+] on day 4 in all treatment groups was significantly increased from baseline by 5 mmol/L (P < 0.001); however, the change in [Na+] was not significantly different across groups (P = 0.7). There was no significant difference in percentage of patients or time to reach [Na+] ≥ 130 or ≥135 mmol/L across the 3 groups. Acute kidney injury and hypokalemia (potassium ≤ 3.0 mmol/L) were more common in patients receiving furosemide.
Limitations
Open-label treatment.
Conclusions
In patients with SIAD, furosemide with NaCl supplement in combination with fluid restriction did not show benefits in correction of [Na+] compared with treatment with fluid restriction alone. Incidences of acute kidney injury and hypokalemia were increased in patients receiving furosemide.
Funding
None.
Trial Registration
Registered at the Thai Clinical Trial Registry with study number TCTR20170629004.
中文翻译:

速尿,口服氯化钠和限液治疗不当抗利尿症(SIAD)综合征的功效:一项开放标签的随机对照研究(EFFUSE-FLUID试验)。
理由与目标
抗利尿不当综合症(SIAD)的一线治疗是限制体液。对于对液体限制没有反应的患者的其他治疗方法是使用速尿限制水或使用速尿和补充盐限制水。但是,从未在随机对照研究中测试过这些疗法的功效。这项研究的目的是研究在补充或不补充氯化钠(NaCl)的情况下速尿联合单独应用液体限制疗法在SIAD低钠血症治疗中是否更有效。
学习规划
开放标签的随机对照研究。
设置与参与者
SIAD导致血钠浓度([Na + ])≤130 mmol / L的患者。
干预措施
随机分配给3组中的1组:单独的限流(FR),限流和速尿(FR + FM),或限流,速尿和NaCl(FR + FM + NaCl)。尿液与血清电解质的比例决定了严格的体液限制(<1,000或<500 mL / d)。速尿剂量为20至40 mg / d。NaCl补充剂为3 g / d。所有治疗持续28天。
结果
主要结局是随机分组后第4、7、14和28天[Na + ]的变化。
结果
招募了92名患者(FR,n = 31; FR + FM,n = 30; FR + FM + NaCl,n = 31)。基线[Na + ]为125±4 mmol / L,两组之间无显着差异。所有治疗组第4天的平均[Na + ]比基线明显增加5 mmol / L(P <0.001);然而,各组间[Na + ]的变化无显着差异(P = 0.7)。在三组中,达到[Na + ]≥130或≥135mmol / L的患者百分比或时间没有显着差异。接受速尿的患者更常见急性肾损伤和低钾血症(钾≤3.0 mmol / L)。
局限性
开放标签治疗。
结论
在SIAD患者中,与单独使用限液治疗相比,加NaCl补充速尿与限液相结合并没有显示出对[Na + ]校正的益处。接受速尿的患者急性肾损伤和低血钾的发生率增加。
资金
没有。
试用注册
在泰国临床试验注册中心注册,研究号为TCTR20170629004。


























 京公网安备 11010802027423号
京公网安备 11010802027423号