Journal of Functional Foods ( IF 5.6 ) Pub Date : 2020-03-18 , DOI: 10.1016/j.jff.2020.103896 Laura Domínguez Díaz , Virginia Fernández-Ruiz , Montaña Cámara
|
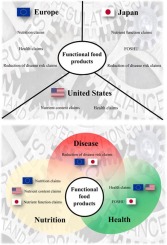
Consumers are increasingly aware of the importance of diet in human health and they preferably choose functional food products. The present work is focused on describing the current status of the international regulatory framework for health-related claims in functional food products and the state of the art regarding these products market focusing on those with health-related claims. Specific regulation must control the use of these claims in the labeling of functional products. Although the European, American and Japanese claims are partly similar in nature, but the approval and use procedures and the regulatory framework are different. Consumers generally accept functional products with health-related claims, and most of them include more than one claim. This review could help consumers for making better-informed food decisions; food industry in marketing its products with a focus on international trade; and scientists in order to put in value their research work.
中文翻译:

功能性食品标签中与食品健康有关的声明的国际法规审查
消费者越来越意识到饮食对人类健康的重要性,因此他们最好选择功能性食品。本工作着重于描述功能性食品中与健康相关的声明的国际监管框架的当前状况,以及针对这些产品市场的最新技术,重点是与健康相关的声明。特殊法规必须控制功能产品标签中这些声明的使用。尽管欧洲,美国和日本的索赔在性质上部分相似,但是批准和使用程序以及监管框架不同。消费者通常会接受带有健康相关声明的功能产品,其中大多数都包含一个以上的声明。这项审查可以帮助消费者做出更明智的食品决策;食品工业以国际贸易为重点的产品营销;和科学家,以珍惜他们的研究工作。



























 京公网安备 11010802027423号
京公网安备 11010802027423号