当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Understanding the Interaction Mechanism between Elemental Selenium and Ferric Hydroxide in Wastewater Treatment
Industrial & Engineering Chemistry Research ( IF 4.2 ) Pub Date : 2020-03-27 , DOI: 10.1021/acs.iecr.0c00533 Jingyi Wang 1 , Junmeng Li 1 , Lei Xie 1 , Qingxia Liu 1 , Hongbo Zeng 1
Industrial & Engineering Chemistry Research ( IF 4.2 ) Pub Date : 2020-03-27 , DOI: 10.1021/acs.iecr.0c00533 Jingyi Wang 1 , Junmeng Li 1 , Lei Xie 1 , Qingxia Liu 1 , Hongbo Zeng 1
Affiliation
|
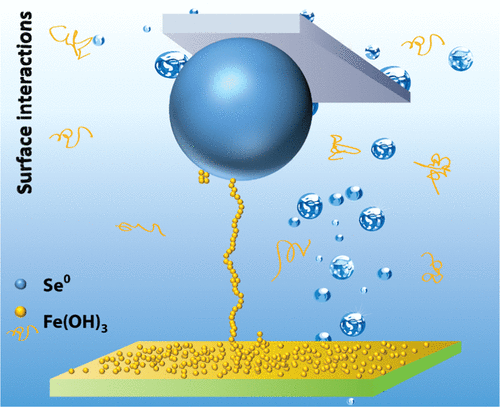
The conversion of selenium oxyanions to elemental selenium (Se0) of low solubility and bioavailability is an effective industrial approach for selenium management in wastewater treatment. The generated Se0 particles require further treatment with coagulants such as ferric ions to facilitate the precipitation of Se0 particles. In this work, the settling of Se0 particles in simulated wastewater was investigated with amorphous ferric hydroxide (Fe(OH)3) as a coagulant prepared from hydrolysis of ferric chloride. The effects of ferric ion dosage, water composition, and pH on the Se0 removal percentage were investigated. The surface properties, i.e., ζ potential and morphology, were characterized, which influenced the interactions between Se0 and Fe(OH)3. The forces acting between Se0 and Fe(OH)3 surfaces were directly measured, for the first time, using atomic force microscopy (AFM). The water composition and pH had a significant effect on the adhesion force. In simulated wastewater, the adhesion force generally increased with pH, suggesting that the adsorption of Ca2+ and Mg2+ on Fe(OH)3 surface increased with pH, which enhanced the adhesion. Interestingly, long-range pull-off forces and sawtooth patterns were observed on the retraction force–separation curves, which were attributed to the stretching of Fe(OH)3 particle aggregates or chains during separation. Bulk settling tests showed that the best Se0 removal performance of Fe(OH)3 was found to be around pH 8, which was because the largest amount of Fe(OH)3 precipitates was found around this pH. The results indicate that the Fe(OH)3 solubility as well as the related intermolecular and surface forces play the predominant role in determining the Se0 removal performance of the ferric coagulant. This work, for the first time, revealed the interaction mechanisms of Se0 particle and amorphous Fe(OH)3, providing useful insights on the performance of ferric coagulants on Se0 particle removal from wastewater under various solution conditions. The experimental approach used in this work can be readily extended to other water treatment systems and processes such as polymer flocculation.
中文翻译:

了解元素硒与氢氧化铁在废水处理中的相互作用机理
低溶解度和生物利用度的硒氧阴离子转化为元素硒(Se 0)是废水处理中硒管理的有效工业方法。产生的Se 0颗粒需要用诸如铁离子的凝结剂进一步处理,以促进Se 0颗粒的沉淀。在这项工作中,使用无定形氢氧化铁(Fe(OH)3)作为由氯化铁水解制得的混凝剂,研究了Se 0颗粒在模拟废水中的沉降。三价铁离子,水的组成和pH对Se 0的影响调查去除率。表征了表面性质,即ζ电势和形态,这影响了Se 0和Fe(OH)3之间的相互作用。使用原子力显微镜(AFM)首次直接测量了在Se 0和Fe(OH)3表面之间作用的力。水的组成和pH值对粘附力有显着影响。在模拟废水中,粘附力通常随pH的增加而增加,表明Ca 2+和Mg 2+在Fe(OH)3上的吸附pH值会增加表面,从而增强附着力。有趣的是,在收缩力-分离曲线上观察到了远距离的拉拔力和锯齿图案,这归因于分离过程中Fe(OH)3颗粒聚集体或链的拉伸。大量沉降试验表明,发现Fe(OH)3的最佳Se 0去除性能约为pH 8,这是因为在该pH值附近发现了最大量的Fe(OH)3沉淀。结果表明,Fe(OH)3的溶解度以及相关的分子间和表面力在确定Se 0方面起主要作用铁凝结剂的去除性能。这项工作首次揭示了Se 0粒子与无定形Fe(OH)3的相互作用机理,提供了关于在各种溶液条件下三聚铁凝结剂对去除废水中Se 0粒子性能的有用见解。在这项工作中使用的实验方法可以很容易地扩展到其他水处理系统和过程,例如聚合物絮凝。
更新日期:2020-03-28
中文翻译:

了解元素硒与氢氧化铁在废水处理中的相互作用机理
低溶解度和生物利用度的硒氧阴离子转化为元素硒(Se 0)是废水处理中硒管理的有效工业方法。产生的Se 0颗粒需要用诸如铁离子的凝结剂进一步处理,以促进Se 0颗粒的沉淀。在这项工作中,使用无定形氢氧化铁(Fe(OH)3)作为由氯化铁水解制得的混凝剂,研究了Se 0颗粒在模拟废水中的沉降。三价铁离子,水的组成和pH对Se 0的影响调查去除率。表征了表面性质,即ζ电势和形态,这影响了Se 0和Fe(OH)3之间的相互作用。使用原子力显微镜(AFM)首次直接测量了在Se 0和Fe(OH)3表面之间作用的力。水的组成和pH值对粘附力有显着影响。在模拟废水中,粘附力通常随pH的增加而增加,表明Ca 2+和Mg 2+在Fe(OH)3上的吸附pH值会增加表面,从而增强附着力。有趣的是,在收缩力-分离曲线上观察到了远距离的拉拔力和锯齿图案,这归因于分离过程中Fe(OH)3颗粒聚集体或链的拉伸。大量沉降试验表明,发现Fe(OH)3的最佳Se 0去除性能约为pH 8,这是因为在该pH值附近发现了最大量的Fe(OH)3沉淀。结果表明,Fe(OH)3的溶解度以及相关的分子间和表面力在确定Se 0方面起主要作用铁凝结剂的去除性能。这项工作首次揭示了Se 0粒子与无定形Fe(OH)3的相互作用机理,提供了关于在各种溶液条件下三聚铁凝结剂对去除废水中Se 0粒子性能的有用见解。在这项工作中使用的实验方法可以很容易地扩展到其他水处理系统和过程,例如聚合物絮凝。


























 京公网安备 11010802027423号
京公网安备 11010802027423号