当前位置:
X-MOL 学术
›
J. Agric. Food Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Multistimuli-Responsive Pickering Emulsion Stabilized by Se-Containing Surfactant-Modified Chitosan
Journal of Agricultural and Food Chemistry ( IF 6.1 ) Pub Date : 2020-03-24 , DOI: 10.1021/acs.jafc.0c00010 Xiaofei Ren 1 , Shuai He 2 , Deqiong Liu 1 , Yongmin Zhang 1
Journal of Agricultural and Food Chemistry ( IF 6.1 ) Pub Date : 2020-03-24 , DOI: 10.1021/acs.jafc.0c00010 Xiaofei Ren 1 , Shuai He 2 , Deqiong Liu 1 , Yongmin Zhang 1
Affiliation
|
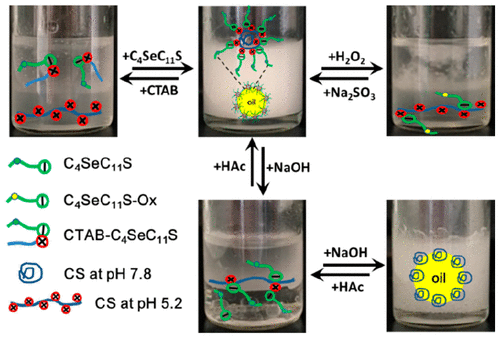
Particle-stabilized emulsions that can respond to external stimuli have attracted significant concerns due to their intelligent-controlled stability, whereas particle-stabilized Pickering emulsions responding to multistimuli but based on biomass have been rarely reported. Here, a multistimuli-responsive Pickering emulsion was developed using the modified chitosan as stabilizer. Due to electrostatic attraction, Se-containing anionic surfactant, sodium 11-(butylselenyl)undecylsulfate (C4SeC11S), can bind with CS at an acidic pH and form CS–C4SeC11S complexes which can further self-associate to form micrometer-sized particles with the character of partially hydrophobicity. Therefore, at pH < pKa, an oil-in-water Pickering emulsion can be formed using CS–C4SeC11S particles as stabilizers and can spontaneously respond to redox, ion, and pH. First, with the addition of oxidation, the hydrophilicity of C4SeC11S was enhanced, and thus, hydrophobic association of CS–C4SeC11S decreased, leading to the disruption of CS–C4SeC11S particles. Hence, the emulsion destabilized. The demulsification process is closely related with the dosage of oxidant and the oxidation time. Second, introduction of a competitive ion (e.g., CTAB) could break the binding between C4SeC11S and CS, leading to the disruption of particle emulsifier. Thereby, demulsification occurred. Third, with sequentially increasing/decreasing pH, the emulsion can be switched from stable to unstable and then to stable again accordingly. Such a unique pH-responsive behavior has never been discovered in other pH-responsive Pickering emulsions. All of the stimuli-responsive behaviors were reversible. Upon alternately adding oxidant/reductant, CTAB/C4SeC11S, or base/acid, the current emulsion can be reversibly switched off (destabilization) and on (stabilization). Such a Pickering emulsion may be a good candidate as a vehicle of functional ingredient.
中文翻译:

含硒表面活性剂改性壳聚糖稳定的多刺激响应性匹克乳液
由于其智能控制的稳定性,可响应外部刺激的颗粒稳定的乳剂引起了广泛的关注,而很少响应基于生物质的多种刺激的颗粒稳定的Pickering乳剂。在这里,使用改性壳聚糖作为稳定剂,开发了一种多刺激响应的Pickering乳液。由于静电吸引,含硒的阴离子表面活性剂11-(丁基硒烯基)十一烷基硫酸钠(C 4 SeC 11 S)可以在酸性pH下与CS结合形成CS–C 4 SeC 11 S复合物,可以进一步自缔合形成具有部分疏水性的微米级颗粒。因此,在pH <p K a时,可以使用CS–C 4 SeC 11 S颗粒作为稳定剂来形成水包油Pickering乳液,并且可以自发响应氧化还原,离子和pH。首先,通过添加氧化作用,C 4 SeC 11 S的亲水性得到增强,因此CS–C 4 SeC 11 S的疏水缔合作用降低,从而导致CS–C 4 SeC 11 S颗粒破裂。因此,乳液不稳定。破乳过程与氧化剂的用量和氧化时间密切相关。其次,引入竞争性离子(例如CTAB)可能会破坏C 4 SeC 11之间的结合S和CS,导致颗粒乳化剂的破坏。由此,发生破乳。第三,随着pH的依次升高/降低,乳液可从稳定状态转变为不稳定状态,然后相应地再次稳定。在其他pH响应性Pickering乳液中从未发现过这种独特的pH响应行为。所有的刺激反应行为都是可逆的。交替添加氧化剂/还原剂,CTAB / C 4 SeC 11 S或碱/酸后,可以可逆地关闭(稳定化)和开启(稳定化)本乳液。这种Pickering乳液可以作为功能成分的媒介物很好的选择。
更新日期:2020-03-24
中文翻译:

含硒表面活性剂改性壳聚糖稳定的多刺激响应性匹克乳液
由于其智能控制的稳定性,可响应外部刺激的颗粒稳定的乳剂引起了广泛的关注,而很少响应基于生物质的多种刺激的颗粒稳定的Pickering乳剂。在这里,使用改性壳聚糖作为稳定剂,开发了一种多刺激响应的Pickering乳液。由于静电吸引,含硒的阴离子表面活性剂11-(丁基硒烯基)十一烷基硫酸钠(C 4 SeC 11 S)可以在酸性pH下与CS结合形成CS–C 4 SeC 11 S复合物,可以进一步自缔合形成具有部分疏水性的微米级颗粒。因此,在pH <p K a时,可以使用CS–C 4 SeC 11 S颗粒作为稳定剂来形成水包油Pickering乳液,并且可以自发响应氧化还原,离子和pH。首先,通过添加氧化作用,C 4 SeC 11 S的亲水性得到增强,因此CS–C 4 SeC 11 S的疏水缔合作用降低,从而导致CS–C 4 SeC 11 S颗粒破裂。因此,乳液不稳定。破乳过程与氧化剂的用量和氧化时间密切相关。其次,引入竞争性离子(例如CTAB)可能会破坏C 4 SeC 11之间的结合S和CS,导致颗粒乳化剂的破坏。由此,发生破乳。第三,随着pH的依次升高/降低,乳液可从稳定状态转变为不稳定状态,然后相应地再次稳定。在其他pH响应性Pickering乳液中从未发现过这种独特的pH响应行为。所有的刺激反应行为都是可逆的。交替添加氧化剂/还原剂,CTAB / C 4 SeC 11 S或碱/酸后,可以可逆地关闭(稳定化)和开启(稳定化)本乳液。这种Pickering乳液可以作为功能成分的媒介物很好的选择。



























 京公网安备 11010802027423号
京公网安备 11010802027423号