当前位置:
X-MOL 学术
›
J. Mater. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
"Watson-Crick G[triple bond, length as m-dash]C"-inspired supramolecular nanodrug of methotrexate and 5-fluorouracil for tumor microenvironment-activatable self-recognizing synergistic chemotherapy.
Journal of Materials Chemistry B ( IF 7 ) Pub Date : 2020-05-06 , DOI: 10.1039/d0tb00468e Meijin Chen 1 , Shiduan Chen 1 , Fukai Zhu 1 , Fanfan Wang 1 , Haina Tian 1 , Zhongxiong Fan 1 , Sunkui Ke 2 , Zhenqing Hou 1 , Yang Li 3
Journal of Materials Chemistry B ( IF 7 ) Pub Date : 2020-05-06 , DOI: 10.1039/d0tb00468e Meijin Chen 1 , Shiduan Chen 1 , Fukai Zhu 1 , Fanfan Wang 1 , Haina Tian 1 , Zhongxiong Fan 1 , Sunkui Ke 2 , Zhenqing Hou 1 , Yang Li 3
Affiliation
|
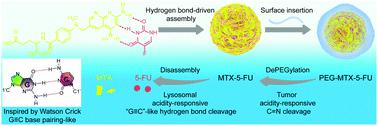
Carrier-free nanodrugs, generated via the straightforward small-molecule self-assembly of anticancer drugs, provide a promising route for cancer chemotherapy. However, their low structural stability, lack of targeting specificity, and poor stimulus responsiveness are still limiting their therapeutic effect. Inspired by Watson-Crick G[triple bond, length as m-dash]C base pairing, the FDA-approved chemo-drug methotrexate (MTX, which can bind with folate receptors) and 5-fluorouracil (5-FU, a DNA/RNA synthetase inhibitor) were adopted for direct assembly into self-recognizing MTX-5-FU nanoparticles via "Watson-Crick-like base pairing"-driven precise supramolecular assembly. Sequentially, our synthesized weak acidity-responsive polyethylene glycol (PEG) was inserted onto the nanoparticle surface to temporarily shield the self-targeting function of MTX and prolong the blood circulation time. Once PEG-MTX-5-FU nanoparticles reached the weakly acidic tumor microenvironment, the PEG corona could be cleaved from their surface and then MTX could be re-exposed to recover its self-recognition ability and significantly elevate tumor cell uptake; furthermore, the de-PEGylated MTX-5-FU nanoparticles could respond to the stronger acidity of lysosome, triggering core disassembly and thus the burst release of both MTX and 5-FU. Further in vitro and in vivo studies consistently confirmed that the nanodrugs exhibited preferable accumulation at the tumor sites with highly synergistic chemotherapeutic effects. The supramolecular recognition-inspired, cascade-triggered self-targeting and controlled release of nanodrugs could be a promising strategy to improve synergistic chemotherapy.
中文翻译:

甲氨蝶呤和5-氟尿嘧啶的“超沃森-克里克三键,长度为m-C”的超分子纳米药物,用于肿瘤微环境可激活的自我识别协同化疗。
通过简单的抗癌药物小分子自组装产生的无载体纳米药物为癌症化学疗法提供了有希望的途径。但是,它们的低结构稳定性,缺乏靶向特异性以及不良的刺激反应性仍然限制了它们的治疗效果。受到Watson-Crick G键的启发,长度为m-C碱基对,是FDA批准的化学药品甲氨蝶呤(MTX,可以与叶酸受体结合)和5-氟尿嘧啶(5-FU,DNA / RNA合成酶抑制剂被用于通过“ Watson-Crick样碱基配对”驱动的精确超分子组装而直接组装成自我识别的MTX-5-FU纳米颗粒。依序,我们将合成的弱酸性响应型聚乙二醇(PEG)插入纳米颗粒表面,以暂时屏蔽MTX的自靶向功能并延长血液循环时间。一旦PEG-MTX-5-FU纳米粒子到达弱酸性肿瘤微环境,就可以将PEG电晕从其表面上裂解下来,然后可以重新暴露MTX以恢复其自我识别能力并显着提高肿瘤细胞的摄取。此外,去PEG化的MTX-5-FU纳米颗粒可以响应溶酶体的强酸性,触发核心分解,从而触发MTX和5-FU的爆发释放。进一步的体外和体内研究一致证实,纳米药物在肿瘤部位表现出优选的蓄积,具有高度协同的化学治疗作用。
更新日期:2020-03-18
中文翻译:

甲氨蝶呤和5-氟尿嘧啶的“超沃森-克里克三键,长度为m-C”的超分子纳米药物,用于肿瘤微环境可激活的自我识别协同化疗。
通过简单的抗癌药物小分子自组装产生的无载体纳米药物为癌症化学疗法提供了有希望的途径。但是,它们的低结构稳定性,缺乏靶向特异性以及不良的刺激反应性仍然限制了它们的治疗效果。受到Watson-Crick G键的启发,长度为m-C碱基对,是FDA批准的化学药品甲氨蝶呤(MTX,可以与叶酸受体结合)和5-氟尿嘧啶(5-FU,DNA / RNA合成酶抑制剂被用于通过“ Watson-Crick样碱基配对”驱动的精确超分子组装而直接组装成自我识别的MTX-5-FU纳米颗粒。依序,我们将合成的弱酸性响应型聚乙二醇(PEG)插入纳米颗粒表面,以暂时屏蔽MTX的自靶向功能并延长血液循环时间。一旦PEG-MTX-5-FU纳米粒子到达弱酸性肿瘤微环境,就可以将PEG电晕从其表面上裂解下来,然后可以重新暴露MTX以恢复其自我识别能力并显着提高肿瘤细胞的摄取。此外,去PEG化的MTX-5-FU纳米颗粒可以响应溶酶体的强酸性,触发核心分解,从而触发MTX和5-FU的爆发释放。进一步的体外和体内研究一致证实,纳米药物在肿瘤部位表现出优选的蓄积,具有高度协同的化学治疗作用。


























 京公网安备 11010802027423号
京公网安备 11010802027423号