当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis and biological evaluation of suffrutines A, B and their N-fused analogues
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2020-03-18 , DOI: 10.1039/d0qo00050g Zefeng Zhu 1, 2, 3, 4 , Chun Chen 1, 2, 3, 4 , Jingxing Jiang 2, 3, 4, 5 , Qianzhong Zhang 1, 2, 3, 4 , Zhibo Du 4, 6, 7, 8 , Shuxian Wei 1, 2, 3, 4 , Xianheng Song 1, 2, 3, 4 , Jie Tang 4, 9, 10 , Jinping Lei 1, 2, 3, 4 , Zhuofeng Ke 2, 3, 4, 5 , Yong Zou 1, 2, 3, 4
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2020-03-18 , DOI: 10.1039/d0qo00050g Zefeng Zhu 1, 2, 3, 4 , Chun Chen 1, 2, 3, 4 , Jingxing Jiang 2, 3, 4, 5 , Qianzhong Zhang 1, 2, 3, 4 , Zhibo Du 4, 6, 7, 8 , Shuxian Wei 1, 2, 3, 4 , Xianheng Song 1, 2, 3, 4 , Jie Tang 4, 9, 10 , Jinping Lei 1, 2, 3, 4 , Zhuofeng Ke 2, 3, 4, 5 , Yong Zou 1, 2, 3, 4
Affiliation
|
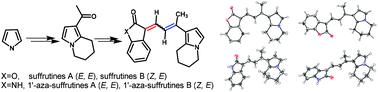
A facile synthetic route to the natural products suffrutines A (1a), B (1b) and their N-fused analogues (2a and 2b) has been accomplished starting from pyrrole in 8 steps. Their structures were elucidated by a series of NMR spectra and single crystal X-ray crystallography. The in situ1H NMR monitoring experiments showed the interconversion properties of the four isomers of suffrutines and their stability order 1a (E, E) > 1b (Z, E) > 1d (Z, Z) > 1c (E, Z) in the solution state under ambient conditions. DFT calculations further confirmed the tautomerization results. The preliminary biological test results showed that 1′-aza-suffrutines B (2b) was the most potent in cytotoxic study (MCF-7, IC50 = 7.31 μM), while 1′-aza-suffrutines A (2a) was the most active in a 14-day colony formation assay. In addition, 2a was proved to be a cancer cell apoptosis inducer in annexin V-FITC/PI dual staining tests.
中文翻译:

A,B及其氮融合类似物的合成及生物学评价
从吡咯开始,从8个步骤开始,已经完成了一种简便的合成途径,可用于合成天然产物Suffrutines A(1a),B(1b)及其N融合类似物(2a和2b)。通过一系列的NMR光谱和单晶X射线晶体学阐明了它们的结构。在原位1个1 H NMR监测实验表明suffrutines的四种异构体的互变的属性和它们的稳定性为了1A(ê,ê)> 1B(Ž,ê)> 1D(Ž,Ž)> 1C(E,Z)在环境条件下处于溶液状态。DFT计算进一步证实了互变异构化结果。初步的生物学测试结果表明,在细胞毒性研究中,1'-氮杂suffrutines B(2b)最有效(MCF-7,IC 50 = 7.31μM),而1'-氮杂suffrutines A(2a)最为有效。在14天的菌落形成试验中具有活性。另外,在膜联蛋白V-FITC / PI双重染色测试中证明2a是癌细胞凋亡诱导剂。
更新日期:2020-03-18
中文翻译:

A,B及其氮融合类似物的合成及生物学评价
从吡咯开始,从8个步骤开始,已经完成了一种简便的合成途径,可用于合成天然产物Suffrutines A(1a),B(1b)及其N融合类似物(2a和2b)。通过一系列的NMR光谱和单晶X射线晶体学阐明了它们的结构。在原位1个1 H NMR监测实验表明suffrutines的四种异构体的互变的属性和它们的稳定性为了1A(ê,ê)> 1B(Ž,ê)> 1D(Ž,Ž)> 1C(E,Z)在环境条件下处于溶液状态。DFT计算进一步证实了互变异构化结果。初步的生物学测试结果表明,在细胞毒性研究中,1'-氮杂suffrutines B(2b)最有效(MCF-7,IC 50 = 7.31μM),而1'-氮杂suffrutines A(2a)最为有效。在14天的菌落形成试验中具有活性。另外,在膜联蛋白V-FITC / PI双重染色测试中证明2a是癌细胞凋亡诱导剂。


























 京公网安备 11010802027423号
京公网安备 11010802027423号