当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Fast surface immobilization of native proteins through catalyst-free amino-yne click bioconjugation
Chemical Science ( IF 8.4 ) Pub Date : 2020-03-18 , DOI: 10.1039/d0sc00062k Yiru Zhang 1 , Jianlei Shen 1 , Rong Hu 1 , Xiujuan Shi 2 , Xianglong Hu 2 , Benzhao He 2 , Anjun Qin 1 , Ben Zhong Tang 1, 2
Chemical Science ( IF 8.4 ) Pub Date : 2020-03-18 , DOI: 10.1039/d0sc00062k Yiru Zhang 1 , Jianlei Shen 1 , Rong Hu 1 , Xiujuan Shi 2 , Xianglong Hu 2 , Benzhao He 2 , Anjun Qin 1 , Ben Zhong Tang 1, 2
Affiliation
|
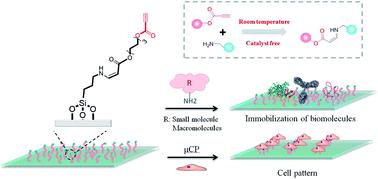
Surface immobilization provides a useful platform for biosensing, drug screening, tissue engineering and other chemical and biological applications. However, some of the used reactions are inefficient and/or complicated, limiting their applications in immobilization. Herein, we use a spontaneous and catalyst-free amino-yne click bioconjugation to generate activated ethynyl group functionalized surfaces for fast immobilization of native proteins and cells. Biomolecules, such as bovine serum albumin (BSA), human IgG and a peptide of C(RGDfK), could be covalently immobilized on the surfaces in as short as 30 min. Notably, the bioactivity of the anchored biomolecules remains intact, which is verified by efficiently capturing target antibodies and cells from the bulk solutions. This strategy represents an alternative for highly efficient surface biofunctionalization.
中文翻译:

通过无催化剂的氨基-炔点击生物共轭快速表面固定天然蛋白质
表面固定为生物传感、药物筛选、组织工程和其他化学和生物应用提供了有用的平台。然而,一些使用的反应效率低下和/或复杂,限制了它们在固定化中的应用。在这里,我们使用自发且无催化剂的氨基-炔点击生物共轭来生成活化的乙炔基功能化表面,以快速固定天然蛋白质和细胞。牛血清白蛋白 (BSA)、人 IgG 和 C(RGDfK) 肽等生物分子可以在短短 30 分钟内共价固定在表面上。值得注意的是,锚定生物分子的生物活性保持完整,这是通过从本体溶液中有效捕获目标抗体和细胞来验证的。该策略代表了高效表面生物功能化的替代方案。
更新日期:2020-04-24
中文翻译:

通过无催化剂的氨基-炔点击生物共轭快速表面固定天然蛋白质
表面固定为生物传感、药物筛选、组织工程和其他化学和生物应用提供了有用的平台。然而,一些使用的反应效率低下和/或复杂,限制了它们在固定化中的应用。在这里,我们使用自发且无催化剂的氨基-炔点击生物共轭来生成活化的乙炔基功能化表面,以快速固定天然蛋白质和细胞。牛血清白蛋白 (BSA)、人 IgG 和 C(RGDfK) 肽等生物分子可以在短短 30 分钟内共价固定在表面上。值得注意的是,锚定生物分子的生物活性保持完整,这是通过从本体溶液中有效捕获目标抗体和细胞来验证的。该策略代表了高效表面生物功能化的替代方案。


























 京公网安备 11010802027423号
京公网安备 11010802027423号