JAMA Dermatology ( IF 10.9 ) Pub Date : 2020-05-01 , DOI: 10.1001/jamadermatol.2020.0352 Debby Wensink 1 , Margreet A E M Wagenmakers 1 , Jasmin Barman-Aksözen 2 , Edith C H Friesema 1 , J H Paul Wilson 1 , Joost van Rosmalen 3 , Janneke G Langendonk 1
|
Importance The effectiveness of afamelanotide treatment in patients with erythropoietic protoporphyria (EPP) in clinical practice who experience pain after light exposure that substantially impairs quality of life is unknown.
Objective To evaluate the association of afamelanotide treatment with outcomes in patients with EPP in regular practice during longer-term follow-up.
Design, Setting, and Participants This single-center, prospective postauthorization safety and efficacy cohort study was directed and approved by the European Medicines Agency. Data were collected from patients with EPP treated with afamelanotide at Erasmus MC between June 2016 and September 2018. Analysis began October 2018.
Main Outcomes and Measures Time spent outside during treatment, number of phototoxic reactions, disease-specific quality of life, usage of protective clothing, and adverse events.
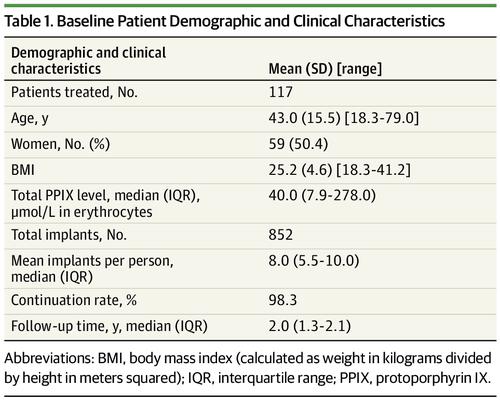
Results A total of 117 patients with EPP (59 women [50.4%]; mean [SD] age, 43.0 [15.5] years) were treated with afamelanotide. Nearly all patients continued treatment (115 [98%]) with a median (interquartile range) follow-up of 2.0 (1.3-2.1) years. Compared with baseline, mean time spent outside during treatment increased significantly by an added 6.1 hours per week (95% CI, 3.62-8.67; P < .001). Mean quality of life score improved significantly by 14.01% (95% CI, 4.53%-23.50%; P < .001). Phototoxic reactions were less painful (β, −0.85; 95% CI, −1.43 to −0.26; P < .001), but there was no significant difference in number or duration of reactions. Minor self-limiting adverse events occurred, such as nausea, fatigue, and headache.
Conclusions and Relevance This cohort study found that afamelanotide treatment was associated with improved clinical outcomes and a good safety profile for patients with EPP. The treatment has clinically significant, sustained positive associations with quality of life, is associated with increased duration of sun exposure, and is associated with less severe phototoxic reactions.
中文翻译:

在临床实践中,Afamelanotide与红细胞原位卟啉症患者改善的结局相关性。
重要性 在临床实践中,阿法莫那肽在患有红细胞生成性原卟啉症(EPP)的患者中的有效性,这些患者在暴露于光后会严重损害生活质量而感到疼痛。
目的 评估长期随访中常规治疗中艾法莫那肽治疗与EPP患者预后的关系。
设计,设置和参与者 这项单中心,前瞻性的授权后安全性和有效性队列研究是由欧洲药品管理局指导和批准的。数据收集自2016年6月至2018年9月在伊拉斯mus斯医学中心接受afamelanotide治疗的EPP患者。分析于2018年10月开始。
主要结果和措施 治疗期间在室外度过的时间,光毒性反应的数量,特定疾病的生活质量,防护服的使用以及不良事件。
结果 共有117例EPP患者(59名女性[50.4%];平均[SD]年龄为43.0 [15.5]岁)接受了阿法美胺治疗。几乎所有患者继续接受治疗(115 [98%]),中位(四分位间距)随访时间为2.0(1.3-2.1)年。与基线相比,治疗期间平均花费在外面的平均时间每周增加了6.1小时(95%CI,3.62-8.67;P <.001)。平均生活质量得分显着提高了14.01%(95%CI,4.53%-23.50%;P <.001)。光毒性反应的痛苦较小(β,-0.85; 95%CI,-1.43至-0.26;P <.001),但反应的数量或持续时间没有显着差异。发生了一些自限性不良事件,例如恶心,疲劳和头痛。
结论与相关性 这项队列研究发现,afamelanotide治疗与EPP患者改善的临床结果和良好的安全性有关。该治疗与生活质量在临床上具有显着的,持续的正相关性,与日光照射的持续时间增加有关,并且与不太严重的光毒性反应有关。


























 京公网安备 11010802027423号
京公网安备 11010802027423号