JAMA Dermatology ( IF 10.9 ) Pub Date : 2020-05-01 , DOI: 10.1001/jamadermatol.2020.0290 Claire Mignard 1 , Maud Maho-Vaillant 1 , Marie-Laure Golinski 1 , Pierre Balayé 2 , Catherine Prost-Squarcioni 3 , Estelle Houivet 2 , Sé Bastien Calbo 1 , Bruno Labeille 4 , Catherine Picard-Dahan 5 , Maria Polina Konstantinou 6 , Guillaume Chaby 7 , Marie-Aleth Richard 8 , Jean-David Bouaziz 9 , Sophie Duvert-Lehembre 10 , Emmanuel Delaporte 10 , Philippe Bernard 11 , Frédéric Caux 3 , Marina Alexandre 3 , Saskia Ingen-Housz-Oro 12 , Pierre Vabres 13 , Gaëlle Quereux 14 , Alain Dupuy 15 , Sébastien Debarbieux 16 , Martine Avenel-Audran 17 , Michel D'Incan 18 , Christophe Bédane 19 , Nathalie Bénéton 20 , Denis Jullien 21 , Nicolas Dupin 22 , Laurent Misery 23 , Laurent Machet 24 , Marie Beylot-Barry 25 , Olivier Dereure 26 , Bruno Sassolas 27 , Jacques Benichou 2 , Pascal Joly 1 , Vivien Hébert 1 ,
|
Importance Rituximab and short-term corticosteroid therapy are the criterion standard treatments for patients with newly diagnosed moderate to severe pemphigus.
Objective To examine factors associated with short-term relapse in patients with pemphigus treated with rituximab.
Design, Setting, and Participants This post hoc analysis of a randomized clinical trial (Comparison Between Rituximab Treatment and Oral Corticosteroid Treatment in Patients With Pemphigus [RITUX 3]) conducted from January 1, 2010, to December 31, 2015, included patients from 20 dermatology departments of tertiary care centers in France from the RITUX 3 trial and 3 newly diagnosed patients treated according to the trial protocol. Data analysis was performed from February 1 to June 30, 2019.
Exposure Patients randomly assigned to the rituximab group in the RITUX 3 trial and the 3 additional patients were treated with 1000 mg of intravenous rituximab on days 0 and 14 and 500 mg at months 12 and 18 combined with a short-term prednisone regimen.
Main Outcomes and Measures Baseline (pretreatment) clinical and biological characteristics (Pemphigus Disease Area Index [PDAI] score, ranging from 0-250 points, with higher values indicating more severe disease) and changes in anti–desmoglein (DSG) 1 and anti-DSG3 values as measured by enzyme-linked immunosorbent assay during the 3 months after rituximab treatment were compared between patients with disease relapse and those who maintained clinical remission during the first 12 months after treatment. The positive and negative predictive values of these factors were calculated.
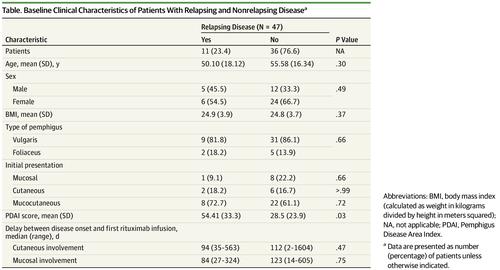
Results Among 47 patients (mean [SD] age, 54.3 [17.0] years; 17 [36%] male and 30 [64%] female) included in the study, the mean (SD) baseline PDAI score for patients with relapsing disease was higher than that of the patients with nonrelapsing disease (54 [33] vs 28 [24]; P = .03). At month 3, 7 of 11 patients with relapsing disease (64%) vs 7 of 36 patients with nonrelapsing disease (19%) had persistent anti-DSG1 antibody values of 20 IU/mL or higher and/or anti-DSG3 antibody values of 130 IU/mL or higher (P = .01). A PDAI score of 45 or higher defining severe pemphigus and/or persistent anti-DSG1 antibody values of 20 IU/mL or higher and/or anti-DSG3 antibody values of 130 IU/mL or higher at month 3 provided a positive predictive value of 50% (95% CI, 27%-73%) and a negative predictive value of 94% (95% CI, 73%-100%) for the occurrence of relapse after rituximab.
Conclusions and Relevance The findings suggest that initial PDAI score and changes in anti-DSG antibody values after the initial cycle of rituximab might help differentiate a subgroup of patients with high risk of relapse who might benefit from maintenance rituximab infusion at month 6 from a subgroup of patients with low risk of relapse who do not need early maintenance therapy.
Trial Registration NCT00784589
中文翻译:

接受利妥昔单抗作为一线治疗的天疱疮患者短期复发相关因素:一项随机临床试验的事后分析。
重要性 利妥昔单抗和短期糖皮质激素治疗是新近诊断为中度至重度天疱疮的患者的标准治疗标准。
目的 探讨利妥昔单抗治疗天疱疮患者短期复发的相关因素。
设计,地点和参与者 这项从2010年1月1日至2015年12月31日进行的随机临床试验(利妥昔单抗治疗与口服皮质类固醇治疗天疱疮[RITUX 3]的比较)的事后分析法国TRIUX 3试验的三级护理中心皮肤科和3名新诊断的患者按照试验方案治疗。数据分析于2019年2月1日至6月30日进行。
暴露 在RITUX 3试验中随机分配至利妥昔单抗组的患者,另外3名患者在第0天和第14天接受1000 mg静脉注射利妥昔单抗治疗,在第12和18个月接受500 mg联合泼尼松短期治疗。
主要结果和措施 基线(预处理)临床和生物学特征(天疱疮疾病面积指数[PDAI]评分,范围为0-250点,数值越高表明疾病越严重)以及抗桥粒芯蛋白(DSG)1和抗比较了利妥昔单抗治疗后3个月内疾病复发患者与治疗后最初12个月内保持临床缓解的患者之间通过酶联免疫吸附法测定的DSG3值。计算这些因素的阳性和阴性预测值。
结果 纳入研究的47例患者(平均[SD]年龄,54.3 [17.0]岁;男性17 [36%]和30 [64%]女性)中,复发性疾病患者的平均(SD)基线PDAI评分为高于非复发性疾病患者(54 [33] vs 28 [24];P = .03)。在第3个月,患有复发性疾病的11名患者中有7名(64%)而非患有复发性疾病的36名患者中有7名(19%)的持续抗DSG1抗体值为20 IU / mL或更高和/或抗DSG3抗体值为130 IU / mL或更高(P = .01)。在第3个月,定义为严重天疱疮的PDAI得分为45或更高,和/或持续抗DSG1抗体值为20 IU / mL或更高,和/或抗DSG3抗体值为130 IU / mL或更高,提供了阳性预测值50%(95%CI,27%-73%)和94%(95%CI,73%-100%)的利妥昔单抗复发复发阴性预测值。
结论与相关性 研究结果表明,最初的PDAI评分和利妥昔单抗初始周期后抗DSG抗体值的变化可能有助于将高复发风险的亚组与那些可能在第6个月接受维持性利妥昔单抗输注的患者从中受益。复发风险低且无需早期维持治疗的患者。
试用注册 NCT00784589


























 京公网安备 11010802027423号
京公网安备 11010802027423号