Chemical Physics Letters ( IF 2.8 ) Pub Date : 2020-03-18 , DOI: 10.1016/j.cplett.2020.137373 Li Wen-Chao , Wang Bao-Rong , Lu Ting , Guo Rui , Li Guang-Yue , Wang Jie-Ping
|
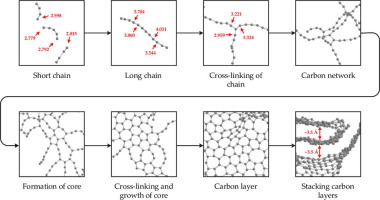
Molecular dynamics based on reactive force field were applied to investigate tar carbonization mechanism. Simulations results revealed that hydrogen-rich gaseous were firstly released and took hydrogen atoms away. At this point, the rest of carbon atoms existed in the form of carbon chains. Then, carbon chains converted to carbon network and even carbon layers by cross-linking reactions. As intermediates, both of them were in a state of matter intermediate between liquid and solid. Especially, formation of aromatic rings offered the growing point for carbon layers. Increasing total bond order of a certain carbon atom pushed the progress of tar carbonization.
中文翻译:

焦油碳化机理的理论分析
基于反作用力场的分子动力学研究焦油碳化机理。模拟结果表明,富氢气态首先被释放并带走了氢原子。此时,其余的碳原子以碳链的形式存在。然后,碳链通过交联反应转化为碳网络甚至碳层。作为中间体,它们两者都处于介于液体和固体之间的物质状态。特别地,芳环的形成为碳层提供了生长点。某个碳原子总键序的增加推动了焦油碳化的进程。


























 京公网安备 11010802027423号
京公网安备 11010802027423号