Catalysis Today ( IF 5.3 ) Pub Date : 2020-03-18 , DOI: 10.1016/j.cattod.2020.03.039 A.J. Reynoso , U. Iriarte-Velasco , M.A. Gutiérrez-Ortiz , J.L. Ayastuy
|
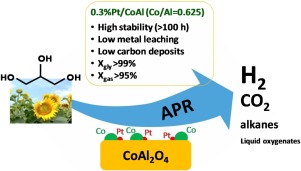
Pt/CoAl2O4 catalysts with small amounts of Pt (0.3 and 1 wt.%) were prepared by wet impregnation of platinum on cobalt aluminate (mole ratio Co/Al = 0.625). These catalysts were compared with monometallic Pt/alumina and cobalt aluminate counterparts. The physicochemical characteristics of the obtained materials were thoroughly analysed. The catalytic performance of the prepared assays was investigated in the Aqueous-Phase Reforming (APR) of glycerol for up to 100 h TOS, and in liquid phase Water-Gas Shift (WGS). It was concluded that the addition of Pt to cobalt aluminate resulted in a synergistic effect that promoted the reduction of both Pt and Co species, as well as cobalt dispersion, what increased the amount of exposed metallic sites. These effects were, however, sensitive to the amount of Pt loaded. The glycerol APR activity of bimetallic catalysts was very stable over 100 h TOS with conversion values above 99%. Conversion to gas was also above 95% during the whole operation. Contrarily, the counterpart monometallic catalysts suffered noticeable deactivation at above 70 h TOS. Also, WGS activity of bimetallic assays was higher than the monometallic counterparts. Addition of Pt to cobalt aluminate lowered selectivity to hydrogen, due to a higher CO hydrogenation activity. Examination of the spent catalysts showed better textural stability of the bimetallic samples, as well as much lesser formation of carbonaceous surface deposits. Nevertheless, oxidation and leaching of cobalt remains as the main drawback of bimetallic catalysts to be used in APR.
中文翻译:

甘油水相重整中高度稳定的Pt / CoAl 2 O 4催化剂
铂/钴2 O 4通过将铂湿浸渍在铝酸钴上(摩尔比Co / Al = 0.625)来制备具有少量Pt(0.3和1 wt。%)的催化剂。将这些催化剂与单金属铂/氧化铝和铝酸钴对应物进行了比较。全面分析了所得材料的理化特性。在甘油的水相重整(APR)中进行长达100小时的TOS以及在液相水煤气变换(WGS)中研究了所制备测定的催化性能。结论是,在铝酸钴中添加Pt产生了协同效应,促进了Pt和Co物种的减少以及钴的分散,从而增加了暴露的金属位点的数量。但是,这些影响对Pt负载量敏感。双金属催化剂的甘油APR活性在100h TOS中非常稳定,转化率超过99%。在整个操作过程中,天然气转化率也高于95%。相反,对应的单金属催化剂在超过70 h TOS时发生了明显的失活。而且,双金属测定的WGS活性高于单金属对应物。由于较高的CO加氢活性,向铝酸钴中添加Pt降低了对氢的选择性。对废催化剂的检查表明双金属样品具有更好的组织稳定性,并且碳质表面沉积物的形成少得多。然而,钴的氧化和浸出仍然是用于APR的双金属催化剂的主要缺点。在整个操作过程中,天然气转化率也高于95%。相反,对应的单金属催化剂在超过70 h TOS时发生了明显的失活。而且,双金属测定的WGS活性高于单金属对应物。由于较高的CO加氢活性,向铝酸钴中添加Pt降低了对氢的选择性。对废催化剂的检查表明双金属样品具有更好的组织稳定性,并且碳质表面沉积物的形成少得多。然而,钴的氧化和浸出仍然是用于APR的双金属催化剂的主要缺点。在整个操作过程中,天然气转化率也高于95%。相反,对应的单金属催化剂在超过70 h TOS时发生了明显的失活。而且,双金属测定的WGS活性高于单金属对应物。由于较高的CO加氢活性,向铝酸钴中添加Pt降低了对氢的选择性。对废催化剂的检查表明双金属样品具有更好的组织稳定性,并且碳质表面沉积物的形成少得多。然而,钴的氧化和浸出仍然是用于APR的双金属催化剂的主要缺点。双金属测定的WGS活性高于单金属对应物。由于较高的CO加氢活性,向铝酸钴中添加Pt降低了对氢的选择性。对废催化剂的检查表明双金属样品具有更好的组织稳定性,并且碳质表面沉积物的形成少得多。然而,钴的氧化和浸出仍然是用于APR的双金属催化剂的主要缺点。双金属测定的WGS活性高于单金属对应物。由于较高的CO加氢活性,向铝酸钴中添加Pt降低了对氢的选择性。对废催化剂的检查表明双金属样品具有更好的组织稳定性,并且碳质表面沉积物的形成少得多。然而,钴的氧化和浸出仍然是用于APR的双金属催化剂的主要缺点。


























 京公网安备 11010802027423号
京公网安备 11010802027423号